Abstract
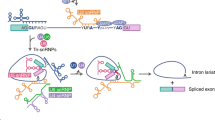
By alternative splicing, different isoforms of the transmembrane tyrosine phosphatase CD45 are generated that either enhance or limit T cell receptor signaling. We report here that CD45 alternative splicing is regulated by cooperative action of the splice factor U2AF26 and the transcription factor Gfi1. U2AF26 promoted formation of the less-active CD45RO by facilitating exon exclusion. Gfi1 antagonized that process by directly interacting with U2AF26, identifying a previously unknown link between a transcription factor and alternative splicing. The presence of Gfi1 led to formation of the more-active CD45RB, whereas loss of Gfi1 favored CD45RO production. We propose that the relative abundance of U2AF26 and Gfi1 determines the ratio of CD45 isoforms, thereby regulating T cell activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72, 291–336 (2003).
Graveley, B.R. Sex, AGility, and the regulation of alternative splicing. Cell 109, 409–412 (2002).
Lynch, K.W. Consequences of regulated pre-mRNA splicing in the immune system. Nat. Rev. Immunol. 4, 931–940 (2004).
Smith, C.W. & Valcarcel, J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci. 25, 381–388 (2000).
Kornblihtt, A.R., de la Mata, M., Fededa, J.P., Munoz, M.J. & Nogues, G. Multiple links between transcription and splicing. RNA 10, 1489–1498 (2004).
Shepard, J., Reick, M., Olson, S. & Graveley, B.R. Characterization of U2AF(6), a splicing factor related to U2AF(35). Mol. Cell. Biol. 22, 221–230 (2002).
Graveley, B.R., Hertel, K.J. & Maniatis, T. The role of U2AF35 and U2AF65 in enhancer-dependent splicing. RNA 7, 806–818 (2001).
Wu, S., Romfo, C.M., Nilsen, T.W. & Green, M.R. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature 402, 832–835 (1999).
Hermiston, M.L., Xu, Z. & Weiss, A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21, 107–137 (2003).
ten Dam, G.B. et al. Regulation of alternative splicing of CD45 by antagonistic effects of SR protein splicing factors. J. Immunol. 164, 5287–5295 (2000).
Byth, K.F. et al. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J. Exp. Med. 183, 1707–1718 (1996).
Mee, P.J. et al. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur. J. Immunol. 29, 2923–2933 (1999).
Penninger, J.M., Irie-Sasaki, J., Sasaki, T. & Oliveira dos Santos, A.J. CD45: new jobs for an old acquaintance. Nat. Immunol. 2, 389–396 (2001).
Mustelin, T. & Tasken, K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem. J. 371, 15–27 (2003).
Birkeland, M.L., Johnson, P., Trowbridge, I.S. & Pure, E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc. Natl. Acad. Sci. USA 86, 6734–6738 (1989).
Lynch, K.W. & Weiss, A. A model system for activation-induced alternative splicing of CD45 pre-mRNA in T cells implicates protein kinase C and Ras. Mol. Cell. Biol. 20, 70–80 (2000).
Xu, Z. & Weiss, A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat. Immunol. 3, 764–771 (2002).
Karsunky, H., Mende, I., Schmidt, T. & Moroy, T. High levels of the onco-protein Gfi-1 accelerate T-cell proliferation and inhibit activation induced T-cell death in Jurkat T-cells. Oncogene 21, 1571–1579 (2002).
Yucel, R., Kosan, C., Heyd, F. & Moroy, T. Gfi1:green fluorescent protein knock-in mutant reveals differential expression and autoregulation of the growth factor independence 1 (Gfi1) gene during lymphocyte development. J. Biol. Chem. 279, 40906–40917 (2004).
Moroy, T. The zinc finger transcription factor growth factor independence 1 (Gfi1). Int. J. Biochem. Cell Biol. 37, 541–546 (2005).
Yucel, R., Karsunky, H., Klein-Hitpass, L. & Moroy, T. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J. Exp. Med. 197, 831–844 (2003).
ten Dam, G.B., Wieringa, B. & Poels, L.G. Alternative splicing of CD45 pre-mRNA is uniquely obedient to conditions in lymphoid cells. Biochim. Biophys. Acta 1446, 317–333 (1999).
Tsujikawa, K., Uchino, Y., Ichijo, T., Furukawa, T. & Yamamoto, H. Detection of CD45ι mRNA in murine Th1 but not Th2 clones. Immunobiology 201, 506–514 (2000).
Karsunky, H. et al. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat. Genet. 30, 295–300 (2002).
Kung, C. et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat. Med. 6, 343–345 (2000).
Jacobsen, M. et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat. Genet. 26, 495–499 (2000).
Guth, S., Tange, T.O., Kellenberger, E. & Valcarcel, J. Dual function for U2AF(35) in AG-dependent pre-mRNA splicing. Mol. Cell. Biol. 21, 7673–7681 (2001).
Rothrock, C.R., House, A.E. & Lynch, K.W. HnRNP L represses exon splicing via a regulated exonic splicing silencer. EMBO J. 24, 2792–2802 (2005).
Tong, A., Nguyen, J. & Lynch, K.W. Differential expression of CD45 isoforms is controlled by the combined activity of basal and inducible splicing-regulatory elements in each of the variable exons. J. Biol. Chem. 280, 38297–38304 (2005).
Deans, J.P. et al. Transient accumulation and subsequent rapid loss of messenger RNA encoding high molecular mass CD45 isoforms after T cell activation. J. Immunol. 148, 1898–1905 (1992).
Majeti, R. et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell 103, 1059–1070 (2000).
Irles, C. et al. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat. Immunol. 4, 189–197 (2003).
Leitenberg, D., Boutin, Y., Lu, D.D. & Bottomly, K. Biochemical association of CD45 with the T cell receptor complex: regulation by CD45 isoform and during T cell activation. Immunity 10, 701–711 (1999).
Dornan, S. et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J. Biol. Chem. 277, 1912–1918 (2002).
Kozieradzki, I. et al. T cell development in mice expressing splice variants of the protein tyrosine phosphatase CD45. J. Immunol. 158, 3130–3139 (1997).
Ogilvy, S. et al. Either of the CD45RB and CD45RO isoforms are effective in restoring T cell, but not B cell, development and function in CD45-null mice. J. Immunol. 171, 1792–1800 (2003).
Chui, D., Ong, C.J., Johnson, P., Teh, H.S. & Marth, J.D. Specific CD45 isoforms differentially regulate T cell receptor signaling. EMBO J. 13, 798–807 (1994).
McNeill, L. et al. CD45 isoforms in T cell signalling and development. Immunol. Lett. 92, 125–134 (2004).
Acknowledgements
We thank I. Spratte, A. Warda and W. Wegrzyn for technical assistance; P. Plessow and T. Civela for animal care; and A. Weiss (University of California, San Francisco, California) and R. Lührmann (Max Planck Institute for Biophysical Chemistry, Gottingen, Germany) for critically reading the manuscript and for suggestions. Supported by the Deutsche Forschungsgemeinschaft (Mo 435/10-4, 10-5), Fonds der Chemischen Industrie, the IFORES Program of the University of Essen Medical School, and Studienstiftung des deutschen Volkes (F.H.).
Author information
Authors and Affiliations
Contributions
G.t.D. provided essential reagents and helped to write the paper; F.H. did the experimental work; F.H. and T.M. designed the experiments, analyzed the data and wrote the paper; and T.M. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Mapping of interaction domains in Gfi1 and U2AF26. (PDF 260 kb)
Supplementary Fig. 2
Real-time PCR for the detection of different CD45 isoforms. (PDF 256 kb)
Supplementary Fig. 3
U2AF26-Flag transgene expression is downregulated after T cell stimulation. (PDF 145 kb)
Supplementary Fig. 4
Phenotype of U2AF26 transgenic T cells was indistinguishable from WT T cells. (PDF 458 kb)
Rights and permissions
About this article
Cite this article
Heyd, F., ten Dam, G. & Möröy, T. Auxiliary splice factor U2AF26 and transcription factor Gfi1 cooperate directly in regulating CD45 alternative splicing. Nat Immunol 7, 859–867 (2006). https://doi.org/10.1038/ni1361
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1361
This article is cited by
-
Zinc finger proteins: insights into the transcriptional and post transcriptional regulation of immune response
Molecular Biology Reports (2021)
-
RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins
Nature Reviews Immunology (2017)
-
Effects of Immunoregulatory Cytokines (IL-2, IL-7, and IL-15) on Expression of Gfi1 and U2afll4 Genes in T Cells at Different Stages of Differentiation
Bulletin of Experimental Biology and Medicine (2015)
-
Gfi1 and Gfi1b: key regulators of hematopoiesis
Leukemia (2010)