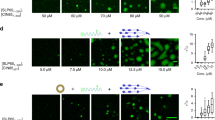
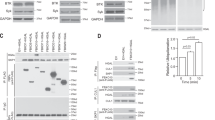
Abstract
Ligation of the B cell antigen receptor (BCR) with antigen induces lipid raft coalescence, a process that occurs after crosslinking of a variety of signaling receptors and is thought to potentiate cellular activation. To investigate lipid raft dynamics during BCR signaling, we quantitatively analyzed the B cell lipid raft proteome. BCR engagement induced dissociation of the adaptor protein ezrin from lipid rafts as well as threonine dephosphorylation of ezrin and its concomitant detachment from actin, indicating a transient uncoupling of lipid rafts from the actin cytoskeleton. Expression of constitutively active ezrin chimeras inhibited the BCR-induced coalescence of lipid rafts. Our data demonstrate that the release of ezrin from lipid rafts acts as a critical trigger that regulates lipid raft dynamics during BCR signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DeFranco, A.L. The complexity of signaling pathways activated by the BCR. Curr. Opin. Immunol. 9, 296–308 (1997).
Stoddart, A. et al. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462 (2002).
Siemasko, K. & Clark, M.R. The control and facilitation of MHC class II antigen processing by the BCR. Curr. Opin. Immunol. 13, 32–36 (2001).
Pierce, S.K. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2, 96–105 (2002).
Brown, D.A. & London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136 (1998).
Kurzchalia, T.V. & Parton, R.G. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11, 424–431 (1999).
Melkonian, K.A., Ostermeyer, A.G., Chen, J.Z., Roth, M.G. & Brown, D.A. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274, 3910–3917 (1999).
Kusumi, A., Koyama-Honda, I. & Suzuki, K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 5, 213–230 (2004).
Cheng, P.C., Dykstra, M.L., Mitchell, R.N. & Pierce, S.K. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190, 1549–1560 (1999).
Petrie, R.J., Schnetkamp, P.P., Patel, K.D., Awasthi-Kalia, M. & Deans, J.P. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 165, 1220–1227 (2000).
Gupta, N. & DeFranco, A.L. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol. Biol. Cell 14, 432–444 (2003).
Guo, B., Kato, R.M., Garcia-Lloret, M., Wahl, M.I. & Rawlings, D.J. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity 13, 243–253 (2000).
Cheng, P.C., Brown, B.K., Song, W. & Pierce, S.K. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J. Immunol. 166, 3693–3701 (2001).
Langhorst, M.F., Reuter, A. & Stuermer, C.A. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell. Mol. Life Sci. 62, 2228–2240 (2005).
Zhou, H., Boyle, R. & Aebersold, R. Quantitative protein analysis by solid phase isotope tagging and mass spectrometry. Methods Mol. Biol. 261, 511–518 (2004).
Gygi, S.P. et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 17, 994–999 (1999).
Yonemura, S. et al. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 140, 885–895 (1998).
Serrador, J.M. et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 91, 4632–4644 (1998).
Tsukita, S. et al. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 126, 391–401 (1994).
Alonso-Lebrero, J.L. et al. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood 95, 2413–2419 (2000).
Kawabuchi, M. et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404, 999–1003 (2000).
Itoh, K. et al. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168, 541–544 (2002).
Louvet-Vallee, S. ERM proteins: from cellular architecture to cell signaling. Biol. Cell. 92, 305–316 (2000).
Huang, L., Wong, T.Y., Lin, R.C. & Furthmayr, H. Replacement of threonine 558, a critical site of phosphorylation of moesin in vivo, with aspartate activates F-actin binding of moesin. Regulation by conformational change. J. Biol. Chem. 274, 12803–12810 (1999).
Zhang, W., Trible, R.P. & Samelson, L.E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9, 239–246 (1998).
Kusumi, A. & Sako, Y. Cell surface organization by the membrane skeleton. Curr. Opin. Cell Biol. 8, 566–574 (1996).
Fujiwara, T., Ritchie, K., Murakoshi, H., Jacobson, K. & Kusumi, A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157, 1071–1081 (2002).
Egawa, T. et al. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr. Biol. 13, 1252–1258 (2003).
Sommer, K. et al. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity 23, 561–574 (2005).
Bretscher, A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr. Opin. Cell Biol. 11, 109–116 (1999).
Lin, J., Miller, M.J. & Shaw, A.S. The c-SMAC: sorting it all out (or in). J. Cell Biol. 170, 177–182 (2005).
Brown, M.J. et al. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood 102, 3890–3899 (2003).
Faure, S. et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat. Immunol. 5, 272–279 (2004).
Tomas, E.M., Chau, T.A. & Madrenas, J. Clustering of a lipid-raft associated pool of ERM proteins at the immunological synapse upon T cell receptor or CD28 ligation. Immunol. Lett. 83, 143–147 (2002).
Allenspach, E.J. et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15, 739–750 (2001).
Cullinan, P., Sperling, A.I. & Burkhardt, J.K. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol. Rev. 189, 111–122 (2002).
Pedrioli, P.G. et al. A common open representation of mass spectrometry data and its application to proteomics research. Nat. Biotechnol. 22, 1459–1466 (2004).
Yates, J.R., III, Eng, J.K., McCormack, A.L. & Schieltz, D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal. Chem. 67, 1426–1436 (1995).
Han, D.K., Eng, J., Zhou, H. & Aebersold, R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nat. Biotechnol. 19, 946–951 (2001).
Keller, A., Nesvizhskii, A.I., Kolker, E. & Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 (2002).
Nesvizhskii, A.I., Keller, A., Kolker, E. & Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 (2003).
Saeki, K., Miura, Y., Aki, D., Kurosaki, T. & Yoshimura, A. The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J. 22, 3015–3026 (2003).
Acknowledgements
We thank D. Barber (University of California at San Francisco) and members of the DeFranco lab for discussions, and C. MacArthur (Howard Hughes Medical Institute, University of California at San Francisco) for cell sorting. Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK068292 to N.G.), the National Institutes of Health (AI20038 to A.L.D. and AI41109 to J.D.W.) and the National Heart, Lung, and Blood Institute (N01-HV-28179 to the Seattle Proteome Center at the Institute for Systems Biology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Regulation of lipid raft coalescence by ezrin. (PDF 660 kb)
Supplementary Fig. 2
Blockade of lipid raft coalesence does not affect upstream signaling events. (PDF 163 kb)
Supplementary Fig. 3
Blockade of lipid raft coalesence does not affect CD69 upregulation. (PDF 276 kb)
Rights and permissions
About this article
Cite this article
Gupta, N., Wollscheid, B., Watts, J. et al. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor–mediated lipid raft dynamics. Nat Immunol 7, 625–633 (2006). https://doi.org/10.1038/ni1337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1337
This article is cited by
-
Executable models of immune signaling pathways in HIV-associated atherosclerosis
npj Systems Biology and Applications (2022)
-
Moesin as a prognostic indicator of lung adenocarcinoma improves prognosis by enhancing immune lymphocyte infiltration
World Journal of Surgical Oncology (2021)
-
Galectin-9 binds IgM-BCR to regulate B cell signaling
Nature Communications (2018)
-
Lipids in the cell: organisation regulates function
Cellular and Molecular Life Sciences (2018)
-
Transitional B cells commit to marginal zone B cell fate by Taok3-mediated surface expression of ADAM10
Nature Immunology (2017)