Abstract
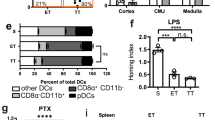
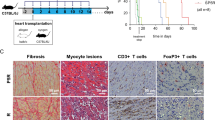
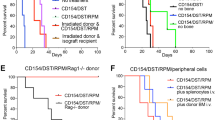
The induction of alloantigen-specific unresponsiveness remains an elusive goal in organ transplantation. Here we identify plasmacytoid dendritic cells (pDCs) as phagocytic antigen-presenting cells essential for tolerance to vascularized cardiac allografts. Tolerizing pDCs acquired alloantigen in the allograft and then moved through the blood to home to peripheral lymph nodes. In the lymph node, alloantigen-presenting pDCs induced the generation of CCR4+CD4+CD25+Foxp3+ regulatory T cells (Treg cells). Depletion of pDCs or prevention of pDC lymph node homing inhibited peripheral Treg cell development and tolerance induction, whereas adoptive transfer of tolerized pDCs induced Treg cell development and prolonged graft survival. Thus, alloantigen-presenting pDCs home to the lymph nodes in tolerogenic conditions, where they mediate alloantigen-specific Treg cell development and allograft tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Krieger, N.R., Yin, D.P. & Fathman, C.G. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 184, 2013–2018 (1996).
Sayegh, M.H. et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am. J. Transplant. 3, 381–389 (2003).
Yamada, A. et al. Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am. J. Transplant. 3, 23–27 (2003).
He, C. & Heeger, P.S. CD8 T cells can reject major histocompatibility complex class I-deficient skin allografts. Am. J. Transplant. 4, 698–704 (2004).
Hancock, W.W. Chemokine receptor-dependent alloresponses. Immunol. Rev. 196, 37–50 (2003).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Sallusto, F. et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 (1998).
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4, 360–370 (2004).
Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6, 345–352 (2005).
Schenk, S. et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+CD25+ T cells. J. Immunol. 174, 3741–3748 (2005).
Hara, M. et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166, 3789–3796 (2001).
Karim, M., Kingsley, C.I., Bushell, A.R., Sawitzki, B.S. & Wood, K.J. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25−CD4+ precursors in a thymus-independent process. J. Immunol. 172, 923–928 (2004).
Cobbold, S.P. et al. Induction of Foxp3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172, 6003–6010 (2004).
Abe, M., Wang, Z., de Creus, A. & Thomson, A.W. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am. J. Transplant. 5, 1808–1819 (2005).
Bai, Y. et al. L-selectin-dependent lymphoid occupancy is required to induce alloantigen-specific tolerance. J. Immunol. 168, 1579–1589 (2002).
Ochando, J.C. et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J. Immunol. 174, 6993–7005 (2005).
Quezada, S.A. et al. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J. Immunol. 175, 771–779 (2005).
Murphy, D.B. et al. A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature 338, 765–768 (1989).
Nakano, H., Yanagita, M. & Gunn, M.D. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194, 1171–1178 (2001).
Grouard, G. et al. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185, 1101–1111 (1997).
Pachynski, R.K., Wu, S.W., Gunn, M.D. & Erle, D.J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin α4β7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 161, 952–956 (1998).
Rosen, S.D. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am. J. Pathol. 155, 1013–1020 (1999).
Berg, E.L., McEvoy, L.M., Berlin, C., Bargatze, R.F. & Butcher, E.C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 366, 695–698 (1993).
Stamper, H.B., Jr & Woodruff, J.J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J. Exp. Med. 144, 828–833 (1976).
Yoneyama, H. et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16, 915–928 (2004).
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Alferink, J. et al. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J. Exp. Med. 197, 585–599 (2003).
Iellem, A. et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194, 847–853 (2001).
Shinohara, M. et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature 416, 759–763 (2002).
Sivalenka, R.R. & Jessberger, R. SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol. Cell. Biol. 24, 10277–10288 (2004).
Asselin-Paturel, C., Brizard, G., Pin, J.J., Briere, F. & Trinchieri, G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171, 6466–6477 (2003).
Cella, M., Facchetti, F., Lanzavecchia, A. & Colonna, M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1, 305–310 (2000).
Jahnsen, F.L. et al. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165, 4062–4068 (2000).
Salio, M., Palmowski, M.J., Atzberger, A., Hermans, I.F. & Cerundolo, V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 199, 567–579 (2004).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
Lakkis, F.G., Arakelov, A., Konieczny, B.T. & Inoue, Y. Immunologic 'ignorance' of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat. Med. 6, 686–688 (2000).
Chen, Y., Demir, Y., Valujskikh, A. & Heeger, P.S. The male minor transplantation antigen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J. Immunol. 171, 6510–6518 (2003).
Reed, A.J. et al. Alloreactive CD4 T cell activation in vivo: an autonomous function of the indirect pathway of alloantigen presentation. J. Immunol. 171, 6502–6509 (2003).
Wagers, A.J. & Kansas, G.S. Potent induction of α(1,3)-fucosyltransferase VII in activated CD4+ T cells by TGF-β1 through a p38 mitogen-activated protein kinase-dependent pathway. J. Immunol. 165, 5011–5016 (2000).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Corbascio, M. et al. CTLA4Ig combined with anti-LFA-1 prolongs cardiac allograft survival indefinitely. Transpl. Immunol. 10, 55–61 (2002).
Kandula, S. & Abraham, C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J. Immunol. 173, 4443–4451 (2004).
Brandt, M., Steinmann, J., Steinhoff, G. & Haverich, A. Treatment with monoclonal antibodies to ICAM-1 and LFA-1 in rat heart allograft rejection. Transpl. Int. 10, 141–144 (1997).
Liu, Y.J., Oldfield, S. & MacLennan, I.C. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur. J. Immunol. 18, 355–362 (1988).
Ellyard, J.I. et al. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood 103, 3805–3812 (2004).
Shapiro-Shelef, M. & Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 5, 230–242 (2005).
Crowley, M., Inaba, K. & Steinman, R.M. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J. Exp. Med. 172, 383–386 (1990).
Josien, R., Heslan, M., Brouard, S., Soulillou, J.P. & Cuturi, M.C. Critical requirement for graft passenger leukocytes in allograft tolerance induced by donor blood transfusion. Blood 92, 4539–4544 (1998).
Baldwin, W.M., III, Rhoton, K. & Sanfilippo, F. IgM and IgG alloantibody production by splenocytes and deposition in rat renal allografts are decreased by donor-specific blood transfusion. Transplantation 51, 481–485 (1991).
Han, S. et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7–2 in established germinal centers. J. Immunol. 155, 556–567 (1995).
de Heer, H.J. et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200, 89–98 (2004).
Itano, A.A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Moseman, E.A. et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173, 4433–4442 (2004).
Kretschmer, K. et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6, 1219–1227 (2005).
Fontenot, J.D. et al. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22, 329–341 (2005).
Corry, R.J., Winn, H.J. & Russell, P.S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16, 343–350 (1973).
Acknowledgements
We acknowledge the technical contributions of J. Llodra, H. Nikolayevskiy and S. Freeman, and discussions with M. Merad. Supported by National Institutes of Health (R01 AI41428, AI44929 and AI62765 to J.S.B.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Real-time PCR analysis of type 1 interferon and Toll-like receptor (TLR) expression of blood circulating YAe+PDCA-1+ cells from tolerized and rejecting mice, compared to pDC from naïve animals (*, P>0.01 by one-way ANOVA) (n=3). (PDF 161 kb)
Supplementary Fig. 2
Phenotypic analyses of the expression of the CD62L ligand MAdCAM1 in HEV from tolerized, rejecting and naïve mice (n=2). (PDF 132 kb)
Supplementary Fig. 3
CCL17 expression in 1-week rejecting LN and splenic YAe+PDCA-1+ and YAe−PDCA-1+ cells (n=3). (PDF 135 kb)
Supplementary Fig. 4
SWAP-70 analysis of CD4+ T cells and CD11c+-B220+-PDCA-1+ cells by immunoblotting from wild-type mice (n=2). (PDF 100 kb)
Supplementary Fig. 5
Total percentage of CD4+ T cells and PDCA-1+ pDC in the LN or spleen of naïve Swap70—/— and wild-type (WT) mice (n=4). (PDF 152 kb)
Supplementary Fig. 6
Total percentage of CD4+ T cells and PDCA-1+ pDC in the LN of Swap70—/— and wild-type (WT) mice following transplantation and tolerogenic treatment with DST + anti-CD40L mAb (n=4), on day of rejection. (PDF 145 kb)
Supplementary Fig. 7
Flow cytometric analysis of the CD4+CD25+ T cell population in Swap70—/— and wild-type (WT) mice following transplantation and tolerogenic treatment with DST + anti-CD40L mAb (n=4), on day of rejection. (PDF 184 kb)
Rights and permissions
About this article
Cite this article
Ochando, J., Homma, C., Yang, Y. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7, 652–662 (2006). https://doi.org/10.1038/ni1333
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1333
This article is cited by
-
Identifying the sensor elements of regulatory T cells in atherosclerosis
Nature Cardiovascular Research (2024)
-
Engineering the lymph node environment promotes antigen-specific efficacy in type 1 diabetes and islet transplantation
Nature Communications (2023)
-
Recreation of an antigen-driven germinal center in vitro by providing B cells with phagocytic antigen
Communications Biology (2023)
-
CXCR4 blockade reduces the severity of murine heart allograft rejection by plasmacytoid dendritic cell-mediated immune regulation
Scientific Reports (2021)
-
Novel Immunomodulatory Approaches for Porcine Islet Xenotransplantation
Current Diabetes Reports (2021)