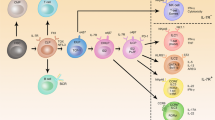
Abstract
The development of lymphoid organs can be viewed as a continuum. At one end are the 'canonical' secondary lymphoid organs, including lymph nodes and spleen; at the other end are 'ectopic' or tertiary lymphoid organs, which are cellular accumulations arising during chronic inflammation by the process of lymphoid neogenesis. Secondary lymphoid organs are genetically 'preprogrammed' and 'prepatterned' during ontogeny, whereas tertiary lymphoid organs arise under environmental influences and are not restricted to specific developmental 'windows' or anatomic locations. Between these two boundaries are other types of lymphoid tissues that are less developmentally but more environmentally regulated, such as Peyer's patches, nasal-associated lymphoid tissue, bronchial-associated lymphoid tissue and inducible bronchial-associated lymphoid tissue. Their regulation, functions and potential effects are discussed here.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Eikelenboom, P., Nassy, J.J., Post, J., Versteeg, J.C. & Langevoort, H.L. The histogenesis of lymph nodes in rat and rabbit. Anat. Rec. 190, 201–215 (1978).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Sabin, F.R. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–389 (1902).
Sabin, F.R. On the development of the superficial lymphatics in the skin of the pig. Am. J. Anat. 3, 183–195 (1904).
Huntington, G.S. & McClure, C.F.W. The anatomy and development of the jugular lymph sac in the domestic cat (Felis Domestica). Am. J. Anat. 10, 177–311 (1910).
Oliver, G. Lymphatic vasculature development. Nat. Rev. Immunol. 4, 35–45 (2004).
Wigle, J.T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Wigle, J.T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Schacht, V. et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 (2003).
Yoshida, H. et al. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167, 2511–2521 (2001).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Yoshida, H. et al. IL-7 receptor α+ CD3− cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 11, 643–655 (1999).
Rennert, P.D., Browning, J.L., Mebius, R., Mackay, F. & Hochman, P.S. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184, 1999–2006 (1996).
Girard, J.P. & Springer, T.A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4, 360–370 (2004).
Gallatin, W.M., Weissman, I.L. & Butcher, E.C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304, 30–34 (1983).
Streeter, P.R., Rouse, B.T. & Butcher, E.C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Hemmerich, S., Butcher, E.C. & Rosen, S.D. Sulfation-dependent recognition of HEV-ligands by L-selectin and MECA-79, an adhesion-blocking mAb. J. Exp. Med. 180, 2219–2226 (1994).
Rosen, S.D. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am. J. Pathol. 155, 1013–1020 (1999).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Bistrup, A. et al. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J. Cell Biol. 145, 899–910 (1999).
Hiraoka, N. et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis(x), an L-selectin ligand displayed by CD34. Immunity 11, 79–89 (1999).
Homeister, J.W. et al. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 (2001).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Mebius, R.E., Streeter, P.R., Michie, S., Butcher, E.C. & Weissman, I.L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+CD3− cells to colonize lymph nodes. Proc. Natl. Acad. Sci. USA 93, 11019–11024 (1996).
Locksley, R.M., Killeen, N. & Lenardo, M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Aggarwal, B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Ware, C.F., Vanarsdale, T.L., Crowe, P.D. & Browning, J.L. The ligands and receptors of the lymphotoxin system. Curr. Top. Microbiol. Immunol. 198, 175–218 (1995).
Browning, J.L. et al. Lymphotoxin-β, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72, 847–856 (1993).
Crowe, P.D. et al. A lymphotoxin-β-specific receptor. Science 264, 707–710 (1994).
Force, W.R. et al. Mouse lymphotoxin-β receptor. Molecular genetics, ligand binding, and expression. J. Immunol. 155, 5280–5288 (1995).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Banks, T.A. et al. Lymphotoxin-α-deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 155, 1685–1693 (1995).
Ying, X., Chan, K., Shenoy, P., Hill, M. & Ruddle, N.H. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am. J. Pathol. 166, 135–146 (2005).
Fukuyama, S. et al. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3−CD4+CD45+ cells. Immunity 17, 31–40 (2002).
Sacca, R., Turley, S., Soong, L., Mellman, I. & Ruddle, N.H. Transgenic expression of lymphotoxin restores lymph nodes to lymphotoxin-α-deficient mice. J. Immunol. 159, 4252–4260 (1997).
Alimzhanov, M.B. et al. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc. Natl. Acad. Sci. USA 94, 9302–9307 (1997).
Koni, P.A. et al. Distinct Roles in lymphoid organogenesis for lymphotoxins α and β in lymphotoxin-β deficient mice. Immunity 6, 491–500 (1997).
Rennert, P.D., Browning, J.L. & Hochman, P.S. Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Int. Immunol. 9, 1627–1639 (1997).
Soderberg, K.A., Linehan, M.M., Ruddle, N.H. & Iwasaki, A. MAdCAM-1 expressing sacral lymph node in the lymphotoxin β-deficient mouse provides a site for immune generation following vaginal herpes simplex virus-2 infection. J. Immunol. 173, 1908–1913 (2004).
Drayton, D.L., Ying, X., Lee, J., Lesslauer, W. & Ruddle, N.H. Ectopic LT αβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 197, 1153–1163 (2003).
Ngo, V.N. et al. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999).
Browning, J.L. et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005).
Randolph, G.J., Angeli, V. & Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Cyster, J.G. Lymphoid organ development and cell migration. Immunol. Rev. 195, 5–14 (2003).
Luther, S.A., Tang, H.L., Hyman, P.L., Farr, A.G. & Cyster, J.G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 97, 12694–12699 (2000).
Saeki, H., Moore, A.M., Brown, M.J. & Hwana, S.T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162, 2472–2475 (1999).
Legler, D.F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Bleul, C.C., Fuhlbrigge, R.C., Casasnovas, J.M., Aiuti, A. & Springer, T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 184, 1101–1109 (1996).
Bouneaud, C., Kourilsky, P. & Bousso, P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity 13, 829–840 (2000).
Hemmi, H. et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-β1-dependent cells. Int. Immunol. 13, 695–704 (2001).
Scheinecker, C., McHugh, R., Shevach, E.M. & Germain, R.N. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196, 1079–1090 (2002).
Huang, F.P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Wilson, N.S. & Villadangos, J.A. Lymphoid organ dendritic cells: beyond the Langerhans cells paradigm. Immunol. Cell Biol. 82, 91–98 (2004).
Wilson, N.S., El-Sukkari, D. & Villadangos, J.A. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 103, 2187–2195 (2004).
Steinman, R.M. et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. NY Acad. Sci. 987, 15–25 (2003).
Cavanagh, L.L. & Von Andrian, U.H. Travellers in many guises: the origins and destinations of dendritic cells. Immunol. Cell Biol. 80, 448–462 (2002).
Wilson, N.S. et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102, 2187–2194 (2003).
Stoitzner, P., Tripp, C.H., Douillard, P., Saeland, S. & Romani, N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J. Invest. Dermatol. 125, 116–125 (2005).
Cupedo, T., Jansen, W., Kraal, G. & Mebius, R.E. Induction of secondary and tertiary lymphoid structures in the skin. Immunity 21, 655–667 (2004).
Hall, J.G., Hopkins, J. & Reynolds, J. Studies of efferent lymph cells from nodes stimulated with oxazolone. Immunology 39, 141–149 (1980).
Hall, J.G. & Smith, M.E. Studies on the afferent and efferent lymph of lymph nodes draining the site of application of fluorodinitrobenzene (FDNB). Immunology 21, 69–79 (1971).
He, C. et al. Stimulation of regional lymphatic and blood flow by epicutaneous oxazolone. J. Appl. Physiol. 93, 966–973 (2002).
West, C.A. et al. Stochastic regulation of cell migration from the efferent lymph to oxazolone-stimulated skin. J. Immunol. 166, 1517–1523 (2001).
Hay, J.B., Cahill, R.N. & Trnka, Z. The kinetics of antigen-reactive cells during lymphocyte recruitment. Cell. Immunol. 10, 145–153 (1974).
Cahill, R.N., Frost, H. & Trnka, Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J. Exp. Med. 143, 870–888 (1976).
Katakai, T., Hara, T., Sugai, M., Gonda, H. & Shimizu, A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200, 783–795 (2004).
Hay, J.B. & Hobbs, B.B. The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J. Exp. Med. 145, 31–44 (1977).
Ottaway, C.A. & Parrott, D.M. Regional blood flow and its relationship to lymphocyte and lymphoblast traffic during a primary immune reaction. J. Exp. Med. 150, 218–230 (1979).
Bai, Y. et al. L-selectin-dependent lymphoid occupancy is required to induce alloantigen-specific tolerance. J. Immunol. 168, 1579–1589 (2002).
Soderberg, K.A. et al. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. USA 102, 16315–16320 (2005).
Myking, A.O. Morphological changes in paracortical high endothelial venules to single and repeated application of oxazolone to mouse skin. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 35, 63–71 (1980).
Mebius, R.E., Breve, J., Duijvestijn, A.M. & Kraal, G. The function of high endothelial venules in mouse lymph nodes stimulated by oxazolone. Immunology 71, 423–427 (1990).
Hoke, D. et al. Selective modulation of the expression of L-selectin ligands by an immune response. Curr. Biol. 5, 670–678 (1995).
Swarte, V.V. et al. Regulation of fucosyltransferase-VII expression in peripheral lymph node high endothelial venules. Eur. J. Immunol. 28, 3040–3047 (1998).
Newberry, R.D. & Lorenz, R.G. Organizing a mucosal defense. Immunol. Rev. 206, 6–21 (2005).
Goeringer, G.C. & Vidic, B. The embryogenesis and anatomy of Waldeyer's ring. Otolaryngol. Clin. North Am. 20, 207–217 (1987).
Harmsen, A. et al. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-alpha (LTα) and retinoic acid receptor-related orphan receptor-γ, but the organization of NALT is LTα dependent. J. Immunol. 168, 986–990 (2002).
Hameleers, D.M., van der Ende, M., Biewenga, J. & Sminia, T. An immunohistochemical study on the postnatal development of rat nasal-associated lymphoid tissue (NALT). Cell Tissue Res. 256, 431–438 (1989).
Constant, S.L. et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Invest. 110, 1441–1448 (2002).
Moyron-Quiroz, J.E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Lorenz, R.G. & Newberry, R.D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. NY Acad. Sci. 1029, 44–57 (2004).
Eberl, G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat. Rev. Immunol. 5, 413–420 (2005).
Finke, D., Acha-Orbea, H., Mattis, A., Lipp, M. & Kraehenbuhl, J. CD4+CD3- cells induce Peyer's patch development: role of α4β1 integrin activation by CXCR5. Immunity 17, 363–373 (2002).
Kratz, A., Campos-Neto, A., Hanson, M.S. & Ruddle, N.H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 183, 1461–1472 (1996).
Yeaman, G.R. et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J. Leukoc. Biol. 61, 427–435 (1997).
Bistrup, A. et al. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope. Am. J. Pathol. 164, 1635–1644 (2004).
Pablos, J.L. et al. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-α/β and TNF-α in cultured endothelial cells. BMC Immunol. 6, 6 (2005).
Kerjaschki, D. et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 15, 603–612 (2004).
Luther, S.A., Ansel, K.M. & Cyster, J.G. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J. Exp. Med. 197, 1191–1198 (2003).
Paavonen, K. et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J. Rheumatol. 29, 39–45 (2002).
Maruyama, K. et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372 (2005).
Cursiefen, C. et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050 (2004).
Baluk, P. et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 115, 247–257 (2005).
Kaiserling, E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology 34, 22–29 (2001).
Heikenwalder, M. et al. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 307, 1107–1110 (2005).
Gause, A. et al. The B lymphocyte in rheumatoid arthritis: analysis of rearranged V kappa genes from B cells infiltrating the synovial membrane. Eur. J. Immunol. 25, 2775–2782 (1995).
Schroder, A.E., Greiner, A., Seyfert, C. & Berek, C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 93, 221–225 (1996).
Dorner, T., Hansen, A., Jacobi, A. & Lipsky, P.E. Immunglobulin repertoire analysis provides new insights into the immunopathogenesis of Sjögren's syndrome. Autoimmun. Rev. 1, 119–124 (2002).
Sims, G.P., Shiono, H., Willcox, N. & Stott, D.I. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J. Immunol. 167, 1935–1944 (2001).
Stott, D.I., Hiepe, F., Hummel, M., Steinhauser, G. & Berek, C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren's syndrome. J. Clin. Invest. 102, 938–946 (1998).
Kim, H.J., Krenn, V., Steinhauser, G. & Berek, C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J. Immunol. 162, 3053–3062 (1999).
McMahon, E.J., Bailey, S.L., Castenada, C.V., Waldner, H. & Miller, S.D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Schrama, D. et al. Targeting of lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 14, 111–121 (2001).
Kaufman, D.L. et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366, 69–72 (1993).
Aguzzi, A. & Heikenwalder, M. Prions, cytokines, and chemokines: a meeting in lymphoid organs. Immunity 22, 145–154 (2005).
Seeger, H. et al. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310, 324–326 (2005).
Hjelmstrom, P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J. Leukoc. Biol. 69, 331–339 (2001).
Wotherspoon, A.C. et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342, 575–577 (1993).
Freni, M.A. et al. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology 22, 389–394 (1995).
Yu, P. et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 5, 141–149 (2004).
Wu, Q. et al. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J. Exp. Med. 193, 1327–1332 (2001).
Fava, R.A. et al. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J. Immunol. 171, 115–126 (2003).
Young, C.L., Adamson, T.C.I., Vaughan, J.H. & Fox, R.I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 27, 32–39 (1984).
Takemura, S. et al. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167, 1072–1080 (2001).
Weyand, C.M., Seyler, T.M. & Goronzy, J.J. B cells in rheumatoid synovitis. Arthritis Res. Ther. 7, S9–12 (2005).
Zvaifler, N.J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv. Immunol. 16, 265–336 (1973).
Tsubaki, T. et al. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin. Exp. Immunol. 141, 363–371 (2005).
Shi, K. et al. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J. Immunol. 166, 650–655 (2001).
Amft, N. et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjögren's syndrome. Arthritis Rheum. 44, 2633–2641 (2001).
Barone, F. et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren's syndrome. Arthritis Rheum. 52, 1773–1784 (2005).
Salomonsson, S. et al. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand. J. Immunol. 55, 336–342 (2002).
Murai, H., Hara, H., Hatae, T., Kobayashi, T. & Watanabe, T. Expression of CD23 in the germinal center of thymus from myasthenia gravis patients. J. Neuroimmunol. 76, 61–69 (1997).
Söderström, N. & Biörklund, A. Organization of the invading lymphoid tissue in human lymphoid thyroiditis. Scand. J. Immunol. 3, 295–301 (1974).
Armengol, M.P. et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am. J. Pathol. 159, 861–873 (2001).
Armengol, M.P. et al. Chemokines determine local lymphoneogenesis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune diseases. J. Immunol. 170, 6320–6328 (2003).
Duijvestijn, A.M. et al. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am. J. Pathol. 130, 147–155 (1988).
Prineas, J.W. Multiple sclerosis: presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science 203, 1123–1125 (1979).
Prineas, J.W. & Wright, R.G. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab. Invest. 38, 409–421 (1978).
Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004).
Pashenkov, M., Soderstrom, M. & Link, H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J. Neuroimmunol. 135, 154–160 (2003).
Carlsen, H.S., Baekkevold, E.S., Morton, H.C., Haraldsen, G. & Brandtzaeg, P. Monocyte-like and mature macrophages produce CXCL13 (B-cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood (2004).
Hanninen, A., Jaakkola, I. & Jalkanen, S. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J. Immunol. 160, 6018–6025 (1998).
Yang, X.D., Sytwu, H.K., McDevitt, H.O. & Michie, S.A. Involvement of β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes 46, 1542–1547 (1997).
Hjelmstrom, P. et al. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am. J. Pathol. 156, 1133–1138 (2000).
Cannella, B., Cross, A.H. & Raine, C.S. Upregulation and coexpression of adhesion molecules correlate with relapsing autoimmune demyelination in the central nervous system. J. Exp. Med. 172, 1521–1524 (1990).
Columba-Cabezas, S., Serafini, B., Ambrosini, E. & Aloisi, F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 13, 38–51 (2003).
Magliozzi, R., Columba-Cabezas, S., Serafini, B. & Aloisi, F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 148, 11–23 (2004).
Mooij, P., de Wit, H.J. & Drexhage, H.A. An excess of dietary iodine accelerates the development of a thyroid-associated lymphoid tissue in autoimmune prone BB rats. Clin. Immunol. Immunopathol. 69, 189–198 (1993).
Katakai, T., Hara, T., Sugai, M., Gonda, H. & Shimizu, A. Th1-biased tertiary lymphoid tissue supported by CXC chemokine ligand 13-producing stromal network in chronic lesions of autoimmune gastritis. J. Immunol. 171, 4359–4368 (2003).
Steere, A.C., Duray, P.H. & Butcher, E.C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 31, 487–495 (1988).
Ghosh, S., Steere, A.C., Stollar, B.D. & Huber, B.T. In situ diversification of the antibody repertoire in chronic Lyme arthritis synovium. J. Immunol. 174, 2860–2869 (2005).
Rupprecht, T.A. et al. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65, 448–450 (2005).
Narayan, K. et al. The nervous system as ectopic germinal center: CXCL13 and IgG in lyme neuroborreliosis. Ann. Neurol. 57, 813–823 (2005).
Hillan, K.J. et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver 19, 509–518 (1999).
Mazzucchelli, L. et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Invest. 104, R49–R54 (1999).
Dogan, A., Du, M., Koulis, A., Briskin, M.J. & Isaacson, P.G. Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am. J. Pathol. 151, 1361–1369 (1997).
Kobayashi, M. et al. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 101, 17807–17812 (2004).
Shomer, N.H., Fox, J.G., Juedes, A.E. & Ruddle, N.H. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect. Immun. 71, 3572–3577 (2003).
Yoneyama, H. et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193, 35–49 (2001).
Vermi, W. et al. Role of dendritic cell-derived CXCL13 in the pathogenesis of Bartonella henselae B-rich granuloma. Blood (2005).
Baddoura, F.K. et al. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am. J. Transplant. 5, 510–516 (2005).
Thaunat, O. et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc. Natl. Acad. Sci. USA 102, 14723–14728 (2005).
Houtkamp, M.A., de Boer, O.J., van der Loos, C.M., van der Wal, A.C. & Becker, A.E. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J. Pathol. 193, 263–269 (2001).
Sacca, R., Cuff, C.A., Lesslauer, W. & Ruddle, N.H. Differential activities of secreted lymphotoxin-α3 and membrane lymphotoxin-α1β2 in lymphotoxin-induced inflammation: critical role of TNF receptor 1 signaling. J. Immunol. 160, 485–491 (1998).
Fan, L., Reilly, C.R., Luo, Y., Dorf, M.E. & Lo, D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J. Immunol. 164, 3955–3959 (2000).
Luther, S.A. et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433 (2002).
Chen, S.C. et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J. Immunol. 168, 1001–1008 (2002).
Martin, A.P. et al. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J. Immunol. 173, 4791–4798 (2004).
Acknowledgements
Supported by the National Institutes of Health (RO1 CA 16885, DK 57731 and AI 44453 to N.H.R. and F31 GM 20919 to D.L.D.) and the Anna Fuller Fund (S.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Drayton, D., Liao, S., Mounzer, R. et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 7, 344–353 (2006). https://doi.org/10.1038/ni1330
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1330
This article is cited by
-
Nanovaccine-based strategies for lymph node targeted delivery and imaging in tumor immunotherapy
Journal of Nanobiotechnology (2023)
-
Prognostic value of tertiary lymphoid structures (TLS) in digestive system cancers: a systematic review and meta-analysis
BMC Cancer (2023)
-
The roles of tertiary lymphoid structures in chronic diseases
Nature Reviews Nephrology (2023)
-
The clinical importance of the host anti-tumour reaction patterns in regional tumour draining lymph nodes in patients with locally advanced resectable gastric cancer: a systematic review and meta-analysis
Gastric Cancer (2023)
-
Tertiary Lymphoid Structures Are Associated with a Favorable Prognosis in High-Grade Serous Ovarian Cancer Patients
Reproductive Sciences (2023)