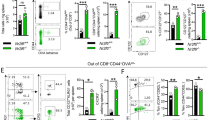
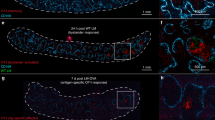
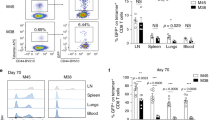
Abstract
Antigen-specific memory T cells are a critical component of protective immunity because of their increased frequency and enhanced reactivity after restimulation. However, it is unclear whether 'memory-like' T cells generated during lymphopenia-induced homeostatic proliferation can also offer protection against pathogens. Here we show that homeostatic proliferation–induced memory (HP-memory) CD8+ T cells controlled bacterial infection as effectively as 'true' memory CD8+ T cells, but their protective capacity required the presence of CD4+ T cells during homeostatic proliferation. The necessity for CD4 help was overcome, however, if the HP-memory CD8+ T cells lacked expression of TRAIL (tumor necrosis factor–related apoptosis-inducing ligand; also called Apo-2L). Thus, like conventional CD8+ memory T cells, the protective function of HP-memory CD8+ T cells shows dependence on CD4+ T cell help.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Masopust, D., Kaech, S.M., Wherry, E.J. & Ahmed, R. The role of programming in memory T-cell development. Curr. Opin. Immunol. 16, 217–225 (2004).
Harty, J.T. & Badovinac, V.P. Influence of effector molecules on the CD8+ T cell response to infection. Curr. Opin. Immunol. 14, 360–365 (2002).
Bourgeois, C., Veiga-Fernandes, H., Joret, A.M., Rocha, B. & Tanchot, C. CD8 lethargy in the absence of CD4 help. Eur. J. Immunol. 32, 2199–2207 (2002).
Bourgeois, C., Rocha, B. & Tanchot, C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297, 2060–2063 (2002).
Janssen, E.M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Shedlock, D.J. & Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300, 337–339 (2003).
Sun, J.C. & Bevan, M.J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Sun, J.C., Williams, M.A. & Bevan, M.J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5, 927–933 (2004).
Ernst, B., Lee, D.-S., Chang, J.M., Sprent, J. & Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Goldrath, A.W. & Bevan, M.J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190 (1999).
Cho, B.K., Rao, V.P., Ge, Q., Eisen, H.N. & Chen, J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192, 549–556 (2000).
Goldrath, A.W., Luckey, C.J., Park, R., Benoist, C. & Mathis, D. The molecular program induced in T cells undergoing homeostatic proliferation. Proc. Natl. Acad. Sci. USA 101, 16885–16890 (2004).
Stockinger, B., Kassiotis, G. & Bourgeois, C. Homeostasis and T cell regulation. Curr. Opin. Immunol. 16, 775–779 (2004).
Marleau, A.M. & Sarvetnick, N. T cell homeostasis in tolerance and immunity. J. Leukoc. Biol. 78, 575–584 (2005).
Marrack, P. et al. Homeostasis of αβ TCR+ T cells. Nat. Immunol. 1, 107–111 (2000).
Almeida, A.R., Rocha, B., Freitas, A.A. & Tanchot, C. Homeostasis of T cell numbers: from thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin. Immunol. 17, 239–249 (2005).
Prlic, M., Blazar, B.R., Khoruts, A., Zell, T. & Jameson, S.C. Homeostatic expansion occurs independently of costimulatory signals. J. Immunol. 167, 5664–5668 (2001).
Hagen, K.A. et al. A role for CD28 in lymphopenia-induced proliferation of CD4 T cells. J. Immunol. 173, 3909–3915 (2004).
Dudley, M.E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Hu, H.-M., Poehlein, C.H., Urba, W.J. & Fox, B.A. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 62, 3914–3919 (2002).
Dummer, W. et al. T cell homeostatic proliferation elicits effective anti-tumor autoimmunity. J. Clin. Invest. 110, 185–192 (2002).
Wrzesinski, C. & Restifo, N.P. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr. Opin. Immunol. 17, 195–201 (2005).
Opferman, J.T., Ober, B.T. & Ashton-Rickardt, P.G. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745–1748 (1999).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
Kaech, S.M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
Kieper, W.C. & Jameson, S.C. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA 96, 13306–13311 (1999).
Goldrath, A.W., Bogatzki, L.Y. & Bevan, M.J. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192, 557–564 (2000).
Murali-Krishna, K. & Ahmed, R. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165, 1733–1737 (2000).
Kieper, W.C., Prlic, M., Schmidt, C.S., Mescher, M.F. & Jameson, S.C. IL-12 enhances CD8 T cell homeostatic expansion. J. Immunol. 166, 5515–5521 (2001).
Oehen, S. & Brduscha-Riem, K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur. J. Immunol. 29, 608–614 (1999).
Tanchot, C., Le Campion, A., Leaument, S., Dautigny, N. & Lucas, B. Naive CD4+ lymphocytes convert to anergic or memory-like cells in T cell-deprived recipients. Eur. J. Immunol. 31, 2256–2265 (2001).
Gavin, M.A., Clarke, S.R., Negrou, E., Gallegos, A. & Rudensky, A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 3, 33–41 (2002).
Janssen, E.M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005).
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–3409 (2001).
Berg, R.E., Crossley, E., Murray, S. & Forman, J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 198, 1583–1593 (2003).
Grusby, M.J., Johnson, R.S., Papaioannou, V.E. & Glimcher, L.H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253, 1417–1420 (1991).
Di Pietro, R. & Zauli, G. Emerging non-apoptotic functions of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/Apo2L. J. Cell. Physiol. 201, 331–340 (2004).
Diehl, G.E. et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity 21, 877–889 (2004).
Harty, J.T., Tvinnereim, A.R. & White, D.W. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18, 275–308 (2000).
Zheng, S.J., Jiang, J., Shen, H. & Chen, Y.H. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J. Immunol. 173, 5652–5658 (2004).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Cretney, E. et al. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 168, 1356–1361 (2002).
Bishop, D.K. & Hinrichs, D.J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139, 2005–2009 (1987).
Daniels, M.A. & Jameson, S.C. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 191, 335–346 (2000).
Acknowledgements
We thank T. Griffith for providing TRAIL-deficient mice; and K. Hogquist, M. Jenkins, M. Mescher and members of the Jamequist lab for input. Supported by the National Institutes of Health (S.C.J. and S.P.S.), the Centers for Disease Control (S.C.J.), the American Cancer Society (S.P.S.), the National Cancer Institute (T32 CA009138 to S.E.H.), the American Cancer Society (S.E.H.) and the Irvington Institute (M.C.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Experimental scheme. (PDF 314 kb)
Supplementary Fig. 2
Protection against LM-OVA by memory OT-I T cells. (PDF 202 kb)
Supplementary Fig. 3
Functional comparison of HP-memory and true memory OT-I T cells. (PDF 782 kb)
Supplementary Fig. 4
HP-memory T cells derived from Tcra−/− hosts do not provide protection against LM-OVA challenge. (PDF 736 kb)
Rights and permissions
About this article
Cite this article
Hamilton, S., Wolkers, M., Schoenberger, S. et al. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol 7, 475–481 (2006). https://doi.org/10.1038/ni1326
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1326
This article is cited by
-
Steady-state memory-phenotype conventional CD4+ T cells exacerbate autoimmune neuroinflammation in a bystander manner via the Bhlhe40/GM-CSF axis
Experimental & Molecular Medicine (2023)
-
Immune response in COVID-19: what is next?
Cell Death & Differentiation (2022)
-
T cell apoptosis characterizes severe Covid-19 disease
Cell Death & Differentiation (2022)
-
Virtual memory CD8 T cells expanded by helminth infection confer broad protection against bacterial infection
Mucosal Immunology (2019)
-
Virtual memory cells make a major contribution to the response of aged influenza-naïve mice to influenza virus infection
Immunity & Ageing (2018)