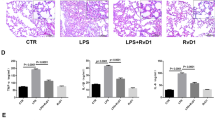
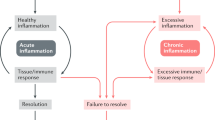
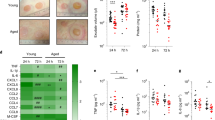
Abstract
Acute inflammation normally resolves by mechanisms that have remained somewhat elusive. Emerging evidence now suggests that an active, coordinated program of resolution initiates in the first few hours after an inflammatory response begins. After entering tissues, granulocytes promote the switch of arachidonic acid–derived prostaglandins and leukotrienes to lipoxins, which initiate the termination sequence. Neutrophil recruitment thus ceases and programmed death by apoptosis is engaged. These events coincide with the biosynthesis, from omega-3 polyunsaturated fatty acids, of resolvins and protectins, which critically shorten the period of neutrophil infiltration by initiating apoptosis. Consequently, apoptotic neutrophils undergo phagocytosis by macrophages, leading to neutrophil clearance and release of anti-inflammatory and reparative cytokines such as transforming growth factor-β1. The anti-inflammatory program ends with the departure of macrophages through the lymphatics. Understanding these and further details of the mechanism required for inflammation resolution may underpin the development of drugs that can resolve inflammatory processes in directed and controlled ways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Majno, G. The Healing Hand: Man and Wound in the Ancient World (Harvard University Press, Cambridge, Massachusetts, 1975).
Gallin, J.I., Snyderman, R., Fearon, D.T., Haynes, B.F. & Nathan, C. Inflammation: Basic Principles and Clinical Correlates (Lippincott Williams & Wilkins, Philadelphia, 1999).
Bannenberg, G.L. et al. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 (2005).
Nathan, C. Points of control in inflammation. Nature 420, 846–852 (2002).
Lawrence, T., Willoughby, D.A. & Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2, 787–795 (2002).
Savill, J. Apoptosis in resolution of inflammation. J. Leukoc. Biol. 61, 375–380 (1997).
Levy, B.D., Clish, C.B., Schmidt, B., Gronert, K. & Serhan, C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 (2001).
Williams, T.J. & Peck, M.J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature 270, 530–532 (1977).
Pouliot, M., Fiset, M.E., Masse, M., Naccache, P.H. & Borgeat, P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J. Immunol. 169, 5279–5286 (2002).
Serhan, C.N. et al. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3 fatty acids via cyclooxygenase2-nonsteroidal anti-inflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 (2000).
Serhan, C.N. et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 196, 1025–1037 (2002).
Gilroy, D.W. et al. Inducible cycloxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 (1999).
Hong, S., Gronert, K., Devchand, P., Moussignac, R.-L. & Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 278, 14677–14687 (2003).
Marcheselli, V.L. et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 278, 43807–43817 (2003).
Serhan, C.N. et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry 34, 14609–14615 (1995).
Chiang, N. et al. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J. Clin. Invest. 104, 309–316 (1999).
Takano, T., Clish, C.B., Gronert, K., Petasis, N. & Serhan, C.N. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 101, 819–826 (1998).
Maddox, J.F. & Serhan, C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J. Exp. Med. 183, 137–146 (1996).
Godson, C. et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 (2000).
Colgan, S.P., Serhan, C.N., Parkos, C.A., Delp-Archer, C. & Madara, J.L. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J. Clin. Invest. 92, 75–82 (1993).
Lawrence, T., Bebien, M., Liu, G.Y., Nizet, V. & Karin, M. IKKα limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434, 1138–1143 (2005).
Savill, J. Apoptosis in post-streptococcal glomerulonephritis. Kidney Int. 60, 1203–1214 (2001).
Metchnikoff, E. Lectures on the Comparative Pathology of Inflammation (Kegan, Paul, Trench and Trubner, London, 1893).
Hurley, J.V. in Acute inflammation (ed. Hurley, J.V.) 109–117 (Churchill Livingstone, London, 1983).
Newman, S.L., Henson, J.E. & Henson, P.M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J. Exp. Med. 156, 430–442 (1982).
Savill, J.S., Wyllie, A.H., Henson, J.E., Walport, M.J. & Henson, P.M. Haslett, C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 (1989).
Bellingan, G.J. et al. In vivo fate of the inflammatory macrophage during the resolution of inflammation: Inflammatory macrophages do not die locally but emigrate to the draining lymph nodes. J. Immunol. 157, 2577–2585 (1996).
Lee, A., Whyte, M.K. & Haslett, C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54, 283–288 (1993).
Ward, C. et al. 1999. NK-κB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 274, 4309 (1999).
Jonsson, H., Allen, P. & Peng, S.L. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat. Med. (2005).
Brown, S.B. Savill, J. Phagocytosis triggers macrophage release of Fas-ligand and induces apoptosis of bystander leukocytes. J. Immunol. 162, 480–485 (1999).
Meagher, L.C., Cousin, J.M. & Seckl, J.R. Haslett, C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 156, 4422 (1996).
Liu, Y. et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 (1999).
Giles, K.M. et al. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation and high levels of active Rac. J. Immunol. 167, 976–986 (2001).
Voll, R.E. et al. Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 (1997).
Fadok, V. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2 and PAF. J. Clin. Invest. 101, 890–898 (1998).
Huynh, M-L.N., Fadok, V.A., Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promoted TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 (2002).
Lucas, M., Stuart, L.M., Savill, J. & Lacy-Hulbert, A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J. Immunol. 171, 2610–2615 (2003).
Byrne, A. & Reen, D.J. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 168, 1968–1997 (2002).
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 (2002).
Lauber, K., Blumenthal, S.G., Waibel, M. & Wesselborg, S. Clearance of apoptotic cells: Getting rid of the corpses. Mol. Cell 14, 277–287 (2004).
Fadok, V. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 (2000).
Bose, J. et al. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J. Biol. 3, 15 (2004).
Duffield, J.S., Ware, C.F., Ryffel, B. & Savill, J. Suppression by apoptotic cells defines tumour necrosis factor-mediated induction of glomerular mesangial cell apoptosis by activated macrophages. Am. J. Pathol. 159, 1397–1404 (2001).
Golpon, H.A. et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 18, 1716–1718 (2004).
Ryoo, H.D., Gorenc, T. & Steller, H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Dev. Cell 7, 491–501 (2004).
Gilmour, J.S. et al. Local amplification of glucocorticoids by 11β-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J. Immunol. (in the press).
Ren, Y. et al. Non-phlogistic clearance of late apoptotic neutrophils by macrophages: Efficient phagocytosis independent of β2 integrins. J. Immunol. 166, 4743–4750 (2001).
Erwig, L.P., Kluth, D.C. & Walsh, G.M. Rees, A.J. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J. Immunol. 161, 1983–1988 (1998).
Erwig, L.P., Stewart, K. & Rees, A.J. Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am. J. Pathol. 156, 295–301 (2000).
Arita, M. et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722 (2005).
Burr, G.O. & Burr, M.M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 82, 345–367 (1929).
GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354, 447–455 (1999).
Marchioli, R. et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 105, 1897–1903 (2002).
Gilroy, D.W. & Perretti, M. Aspirin and steroids: new mechanistic findings and avenues for drug discovery. Curr. Opin. Pharmacol. 5, 405–411 (2005).
Maderna, P., Yona, S., Perretti, M. & Godson, C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from Dexamethasone-treated macrophages and annexin-dervied peptide Ac2–261. J. Immunol. 174, 3727–3733 (2005).
Ward, C. et al. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of IκBα degradation using a peroxisome proliferator-activated receptor-γ-independent mechanism. J. Immunol. 168, 6232–6243 (2002).
Arita, M. et al. Resolvin E1, a novel endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against TNBS-induced colitis. Proc. Natl. Acad. Sci. USA 102, 7671–7676 (2005).
Wallace, J.L. & Fiorucci, S. A magic bullet for mucosal protection...and aspirin is the trigger! Trends Pharmacol. Sci. 24, 323–326 (2003).
Fukunaga, K., Kohli, P., Bonnans, C., Fredenburgh, L.E. & Levy, B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 174, 5033–5039 (2005).
Gilroy, D.W., Lawrence, T., Perretti, M. & Rossi, A.G. Inflammation resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 (2004).
Clària, J. & Serhan, C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA 92, 9475–9479 (1995).
Schottelius, A.J. et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J. Immunol. 169, 7063–7070 (2002).
Fiorucci, S. et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc. Natl. Acad. Sci. USA 101, 15736–15741 (2004).
Karp, C.L. et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 5, 388–392 (2004).
Mitchell, S. et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J. Am. Soc. Nephrol. 13, 2497–2507 (2002).
Taylor, P.R. et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 192, 359–366 (2000).
Hanayama, R. et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147–1150 (2004).
Devitt, A. et al. Persistence of apoptotic cells without autimmune disease or inflammation in CD14−/− mice. J. Cell Biol. 167, 1161–1170 (2004).
Stuart, L.M., Takahashi, K., Shi, L., Savill, J. & Ezekowitz, R.A.B. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotyope. J. Immunol. 174, 3220–3226 (2005).
Samuelsson, B., Dahlén, S.E., Lindgren, J.Å., Rouzer, C.A. & Serhan, C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237, 1171–1176 (1987).
Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 294, 1871–1875 (2001).
Capdevila, J.H., Falck, J.R., Dishman, E. & Karara, A. in Arachidonate Related Lipid Mediators (eds. Murphy, R.C. & Fitzpatrick, F.A.) 385–394 (Academic, San Diego, 1990).
Node, K. et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285, 1276–1279 (1999).
Mukherjee, P.K., Marcheselli, V.L., Serhan, C.N. & Bazan, N.G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 101, 8491–8496 (2004).
Hughes, J.A. & Savill, J. Apoptosis in glomerulonephritis. Cur. Opn. Neph. & Hyper. 14, 389–395 (2005).
Acknowledgements
We thank M.H. Small, C. Gilchrist and C. Law for assistance in manuscript preparation, and K. Gotlinger for assistance with the illustrations. Supported by the National Institutes of Health (P50-DE016191 and GM38765 to C.N.S.), the Wellcome Trust (064487 to J.S.) and the Medical Research Council (J.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Serhan, C., Savill, J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6, 1191–1197 (2005). https://doi.org/10.1038/ni1276
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1276
This article is cited by
-
Stress-induced stenotic vascular remodeling via reduction of plasma omega-3 fatty acid metabolite 4-oxoDHA by noradrenaline
Scientific Reports (2024)
-
Interface between Resolvins and Efferocytosis in Health and Disease
Cell Biochemistry and Biophysics (2024)
-
The impact of novel inflammation-preserving treatment towards lumbar disc herniation resorption in symptomatic patients: a prospective, multi-imaging and clinical outcomes study
European Spine Journal (2024)
-
Network analyses reveal new insights into the effect of multicomponent Tr14 compared to single-component diclofenac in an acute inflammation model
Journal of Inflammation (2023)
-
Fatty acid desaturation and lipoxygenase pathways support trained immunity
Nature Communications (2023)