Abstract
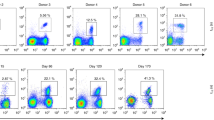
Using both 'reverse genetics' and structural analysis, we have examined the in vivo relationship between antigenicity and T cell receptor (TCR) repertoire diversity. Influenza A virus infection of C57BL/6 mice induces profoundly different TCR repertoires specific for the nucleoprotein peptide of amino acids 366–374 (NP366) and the acid polymerase peptide of amino acids 224–233 (PA224) presented by H-2Db. Here we show the H-2Db–NP366 complex with a 'featureless' structure selected a limited TCR repertoire characterized by 'public' TCR usage. In contrast, the prominent H-2Db–PA224 complex selected diverse, individually 'private' TCR repertoires. Substitution of the arginine at position 7 of PA224 with an alanine reduced the accessible side chains of the epitope. Infection with an engineered virus containing a mutation at the site encoding the exposed arginine at position 7 of PA224 selected a restricted TCR repertoire similar in diversity to that of the H-2Db–NP366–specific response. Thus, the lack of prominent features in an antigenic complex of peptide and major histocompatibility complex class I is associated with a diminished spectrum of TCR usage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davis, M.M. & Chen, Y.H. T cell antigen receptors. in Fundamental Immunology (ed. Paul, W.E.) 341–366 (Lippincott-Raven, Philidelphia, 1999).
Garboczi, D.N. et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384, 134–141 (1996).
Garcia, K.C. et al. An αβ T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science 274, 209–219 (1996).
Jorgensen, J.L., Esser, U., Fazekas de St Groth, B., Reay, P.A. & Davis, M.M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature 355, 224–230 (1992).
Kjer-Nielsen, L. et al. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity 18, 53–64 (2003).
Stewart-Jones, G.B., McMichael, A.J., Bell, J.I., Stuart, D.I. & Jones, E.Y. A structural basis for immunodominant human T cell receptor recognition. Nat. Immunol. 4, 657–663 (2003).
Turner, S.J., Jameson, S.C. & Carbone, F.R. Functional mapping of the orientation for TCR recognition of an H2-Kb-restricted ovalbumin peptide suggests that the β-chain subunit can dominate the determination of peptide side chain specificity. J. Immunol. 159, 2312–2317 (1997).
Wang, F. et al. On defining the rules for interactions between the T cell receptor and its ligand: a critical role for a specific amino acid residue of the T cell receptor β chain. Proc. Natl. Acad. Sci. USA 95, 5217–5222 (1998).
Aebischer, T., Oehen, S. & Hengartner, H. Preferential usage of Vα4 and Vβ10 T cell receptor genes by lymphocytic choriomeningitis virus glycoprotein-specific H-2Db-restricted cytotoxic T cells. Eur. J. Immunol. 20, 523–531 (1990).
Deckhut, A.M. et al. Prominent usage of Vβ8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J. Immunol. 151, 2658–2666 (1993).
Kelly, J.M. et al. Identification of conserved T cell receptor CDR3 residues contacting known exposed peptide side chains from a major histocompatibility complex class I-bound determinant. Eur. J. Immunol. 23, 3318–3326 (1993).
Pantaleo, G. et al. Major expansion of CD8+ T cells with a predominant Vβ usage during the primary immune response to HIV. Nature 370, 463–467 (1994).
Maryanski, J.L. et al. The diversity of antigen-specific TCR repertoires reflects the relative complexity of epitopes recognized. Hum. Immunol. 54, 117–128 (1997).
Yanagi, Y., Maekawa, R., Cook, T., Kanagawa, O. & Oldstone, M.B. Restricted V-segment usage in T-cell receptors from cytotoxic T lymphocytes specific for a major epitope of lymphocytic choriomeningitis virus. J. Virol. 64, 5919–5926 (1990).
Argaet, V.P. et al. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J. Exp. Med. 180, 2335–2340 (1994).
Cibotti, R. et al. Public and private V beta T cell receptor repertoires against hen egg white lysozyme (HEL) in nontransgenic versus HEL transgenic mice. J. Exp. Med. 180, 861–872 (1994).
Burrows, S.R. et al. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J. Exp. Med. 182, 1703–1715 (1995).
Lehner, P.J. et al. Human HLA-A0201-restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T cells bearing the Vβ17 gene segment. J. Exp. Med. 181, 79–91 (1995).
Turner, S.J., Cose, S.C. & Carbone, F.R. TCR α-chain usage can determine antigen-selected TCR beta-chain repertoire diversity. J. Immunol. 157, 4979–4985 (1996).
Callan, M.F. et al. T cell selection during the evolution of CD8+ T cell memory in vivo. Eur. J. Immunol. 28, 4382–4390 (1998).
Moss, P.A. et al. Extensive conservation of α and β chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc. Natl. Acad. Sci. USA 88, 8987–8990 (1991).
Chen, W., Bennink, J.R., Morton, P.A. & Yewdell, J.W. Mice deficient in perforin, CD4+ T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J. Virol. 76, 10332–10337 (2002).
Townsend, A.R. et al. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44, 959–968 (1986).
Belz, G.T., Xie, W., Altman, J.D. & Doherty, P.C. A previously unrecognized H-2Db-restricted peptide prominent in the primary influenza A virus-specific CD8+ T-cell response is much less apparent following secondary challenge. J. Virol. 74, 3486–3493 (2000).
Marshall, D.R. et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 98, 6313–6318 (2001).
Kedzierska, K., Turner, S.J. & Doherty, P.C. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc. Natl. Acad. Sci. USA 101, 4942–4947 (2004).
Crowe, S.R. et al. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 198, 399–410 (2003).
Belz, G.T., Xie, W. & Doherty, P.C. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J. Immunol. 166, 4627–4633 (2001).
La Gruta, N.L., Turner, S.J. & Doherty, P.C. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 172, 5553–5560 (2004).
Flynn, K.J. et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8, 683–691 (1998).
Belz, G.T., Stevenson, P.G. & Doherty, P.C. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 165, 2404–2409 (2000).
Turner, S.J., Diaz, G., Cross, R. & Doherty, P.C. Analysis of clonotype distribution and persistence for an influenza virus-specific CD8+ T cell response. Immunity 18, 549–559 (2003).
Young, A.C., Zhang, W., Sacchettini, J.C. & Nathenson, S.G. The three-dimensional structure of H-2Db at 2.4 Å resolution: implications for antigen-determinant selection. Cell 76, 39–50 (1994).
Turner, S.J., La Gruta, N.L., Stambas, J., Diaz, G. & Doherty, P.C. Differential tumor necrosis factor receptor 2-mediated editing of virus-specific CD8+ effector T cells. Proc. Natl. Acad. Sci. USA 101, 3545–3550 (2004).
Webby, R.J. et al. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc. Natl. Acad. Sci. USA 100, 7235–7240 (2003).
Turner, S.J., Kedzierska, K., La Gruta, N.L., Webby, R. & Doherty, P.C. Characterization of CD8+ T cell repertoire diversity and persistence in the influenza A virus model of localized, transient infection. Semin. Immunol. 16, 179–184 (2004).
Plebanski, M. et al. Altered peptide ligands narrow the repertoire of cellular immune responses by interfering with T-cell priming. Nat. Med. 5, 565–571 (1999).
Man, S., Ridge, J.P. & Engelhard, V.H. Diversity and dominance among TCR recognizing HLA-A2.1+ influenza matrix peptide in human MHC class I transgenic mice. J. Immunol. 153, 4458–4467 (1994).
Messaoudi, I., Guevara Patino, J.A., Dyall, R., LeMaoult, J. & Nikolich-Zugich, J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science 298, 1797–1800 (2002).
Webb, A.I. et al. The structure of H-2Kb and Kbm8 complexed to a herpes simplex virus determinant: evidence for a conformational switch that governs T cell repertoire selection and viral resistance. J. Immunol. 173, 402–409 (2004).
Hoffmann, E., Neumann, G., Kawaoka, Y., Hobom, G. & Webster, R.G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 97, 6108–6113 (2000).
Hoffmann, E., Stech, J., Guan, Y., Webster, R.G. & Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146, 2275–2289 (2001).
Macdonald, W. et al. Identification of a dominant self-ligand bound to three HLA B44 alleles and the preliminary crystallographic analysis of recombinant forms of each complex. FEBS Lett. 527, 27–32 (2002).
Zhao, R., Loftus, D.J., Appella, E. & Collins, E.J. Structural evidence of T cell xeno-reactivity in the absence of molecular mimicry. J. Exp. Med. 189, 359–370 (1999).
Brunger, A.T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Gascoigne, N.R., Chien, Y., Becker, D.M., Kavaler, J. & Davis, M.M. Genomic organization and sequence of T-cell receptor β-chain constant- and joining-region genes. Nature 310, 387–391 (1984).
Chothia, C., Boswell, D.R. & Lesk, A.M. The outline structure of the T-cell αβ receptor. EMBO J. 7, 3745–3755 (1988).
Acknowledgements
The authors thank J. Stambas for critical review of this manuscript, and D. Stockwell and Y. Sims for technical assistance. Supported by a Burnet Fellowship (P.C.D.) and a Peter Doherty Postdoctoral fellowship (K.K) from the Australian National Health and Medical Research Council, a Wellcome Trust Senior Research Fellowship (J.R.), Science, Technology and Innovation (Government of Victoria, Australia), Australian Research Council, National Health and Medical Research Council, Juvenile Diabetes Research Foundation, United States Public Health Service (AI29579) and American Lebanese Syrian Associated Charities at St. Jude Children's Research Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Lung viral titers of mice infected with either HKx31 or PA-R7 viruses. (PDF 50 kb)
Supplementary Fig. 2
Narrowing of PA-R7A TCR repertoire diversity after secondary challenge. (PDF 51 kb)
Supplementary Fig. 3
Primer sequences. (PDF 46 kb)
Rights and permissions
About this article
Cite this article
Turner, S., Kedzierska, K., Komodromou, H. et al. Lack of prominent peptide–major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nat Immunol 6, 382–389 (2005). https://doi.org/10.1038/ni1175
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1175
This article is cited by
-
Covalent TCR-peptide-MHC interactions induce T cell activation and redirect T cell fate in the thymus
Nature Communications (2022)
-
Destabilizing single chain major histocompatibility complex class I protein for repurposed enterokinase proteolysis
Scientific Reports (2020)
-
Divergent T-cell receptor recognition modes of a HLA-I restricted extended tumour-associated peptide
Nature Communications (2018)
-
Broad TCR repertoire and diverse structural solutions for recognition of an immunodominant CD8+ T cell epitope
Nature Structural & Molecular Biology (2017)
-
Crystal structure of HLA-B*5801 with a TW10 HIV Gag epitope reveals a novel mode of peptide presentation
Cellular & Molecular Immunology (2017)