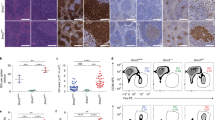
Abstract
It is unknown how B cells that mature during a germinal center reaction 'decide' between plasma or memory cell fate. Here we describe a previously unknown subpopulation of B cells in the human germinal center that is characterized by tyrosine phosphorylated transcriptional activator STAT5. These cells had an activated centrocyte phenotype and had abundant expression of BCL6 but low expression of PRDM1, both encoding transcriptional repression proteins. Using RNA interference and ectopic expression of constitutively activated forms of STAT5, we demonstrate here a function for STAT5 in the self-renewal of B cells in vitro. STAT5b isoform seemed to directly upregulate Bcl-6, and ectopic expression of Bcl-6 in B cells resulted in self-renewal and inhibition of plasma cell differentiation. These data indicate that activation of STAT5 is involved in regulation of memory B cell differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Klein, U. et al. Transcriptional analysis of the B cell germinal center reaction. Proc. Natl. Acad. Sci. USA 100, 2639–2644 (2003).
Calame, K.L., Lin, K.I. & Tunyaplin, C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 21, 205–230 (2003).
Tarlinton, D.M. & Smith, K.G. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol. Today 21, 436–441 (2000).
Stuber, E. & Strober, W. The T cell-B cell interaction via OX40–OX40L is necessary for the T cell-dependent humoral immune response. J. Exp. Med. 183, 979–989 (1996).
Turner, C.A., Jr., Mack, D.H. & Davis, M.M. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 77, 297–306 (1994).
Reljic, R., Wagner, S.D., Peakman, L.J. & Fearon, D.T. Suppression of signal transducer and activator of transcription 3- dependent B lymphocyte terminal differentiation by BCL-6. J. Exp. Med. 192, 1841–1848 (2000).
Shaffer, A.L. et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13, 199–212 (2000).
Shaffer, A.L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Reimold, A.M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Arpin, C. et al. Generation of memory B cells and plasma cells in vitro. Science 268, 720–722 (1995).
Arpin, C., Banchereau, J. & Liu, Y.J. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J. Exp. Med. 186, 931–940 (1997).
Shvarts, A. et al. A senescence rescue screen identifies BCL6 as an inhibitor of anti- proliferative p19(ARF)-p53 signaling. Genes Dev. 16, 681–686 (2002).
Fearon, D.T., Manders, P. & Wagner, S.D. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science 293, 248–250 (2001).
Lischke, A. et al. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J. Biol. Chem. 273, 31222–31229 (1998).
Rolling, C., Treton, D., Pellegrini, S., Galanaud, P. & Richard, Y. IL4 and IL13 receptors share the γ c chain and activate STAT6, STAT3 and STAT5 proteins in normal human B cells. FEBS Lett. 393, 53–56 (1996).
Leonard, W.J. & O'Shea, J.J. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16, 293–322 (1998).
Shimoda, K. et al. Lack of IL-4-induced TH2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380, 630–633 (1996).
Kaplan, M.H., Schindler, U., Smiley, S.T. & Grusby, M.J. STAT6 is required for mediating responses to IL-4 and for development of TH2 cells. Immunity 4, 313–319 (1996).
Teglund, S. et al. STAT5a and STAT5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850 (1998).
Bunting, K.D. et al. Reduced lymphomyeloid repopulating activity from adult bone marrow and fetal liver of mice lacking expression of STAT5. Blood 99, 479–487 (2002).
Sexl, V. et al. STAT5a/b contribute to interleukin 7-induced B-cell precursor expansion, but abl- and bcr/abl-induced transformation are independent of stat5. Blood 96, 2277–2283 (2000).
Burchill, M.A. et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol. 171, 5853–5864 (2003).
Lord, J.D., McIntosh, B.C., Greenberg, P.D. & Nelson, B.H. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of STAT5. J. Immunol. 164, 2533–2541 (2000).
John, S., Robbins, C.M. & Leonard, W.J. An IL-2 response element in the human IL-2 receptor alpha chain promoter is a composite element that binds STAT5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO J. 15, 5627–5635 (1996).
Martinez-Valdez, H. et al. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J. Exp. Med. 183, 971–977 (1996).
Angelin-Duclos, C., Cattoretti, G., Lin, K.I. & Calame, K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 165, 5462–5471 (2000).
Tunyaplin, C. et al. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J. Immunol. 173, 1158–1165 (2004).
Banchereau, J., de Paoli, P., Valle, A., Garcia, E. & Rousset, F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science 251, 70–72 (1991).
Kurata, H., Lee, H.J., O'Garra, A. & Arai, N. Ectopic expression of activated Stat6 induces the expression of TH2- specific cytokines and transcription factors in developing TH1 cells. Immunity 11, 677–688 (1999).
Ohashi, K., Miki, T., Hirosawa, S. & Aoki, N. Characterization of the promoter region of human BCL-6 gene. Biochem. Biophys. Res. Commun. 214, 461–467 (1995).
Wolf, J. et al. Peripheral blood mononuclear cells of a patient with advanced Hodgkin's lymphoma give rise to permanently growing Hodgkin-Reed Sternberg cells. Blood 87, 3418–3428 (1996).
Hinz, M. et al. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin's disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J. Exp. Med. 196, 605–617 (2002).
Raman, V.S., Akondy, R.S., Rath, S., Bal, V. & George, A. Ligation of CD27 on B cells in vivo during primary immunization enhances commitment to memory B cell responses. J. Immunol. 171, 5876–5881 (2003).
Raman, V.S., Bal, V., Rath, S. & George, A. Ligation of CD27 on murine B cells responding to T-dependent and T-independent stimuli inhibits the generation of plasma cells. J. Immunol. 165, 6809–6815 (2000).
Andjelic, S. et al. Phosphatidylinositol 3-kinase and NF-κB/Rel are at the divergence of CD40-mediated proliferation and survival pathways. J. Immunol. 165, 3860–3867 (2000).
Dadgostar, H. et al. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proc. Natl. Acad. Sci. USA 99, 1497–1502 (2002).
Ehret, G.B. et al. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J. Biol. Chem. 276, 6675–6688 (2001).
Lin, Y., Wong, K. & Calame, K. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science 276, 596–599 (1997).
Usui, T. et al. Overexpression of B cell-specific activator protein (BSAP/Pax-5) in a late B cell is sufficient to suppress differentiation to an Ig high producer cell with plasma cell phenotype. J. Immunol. 158, 3197–3204 (1997).
Reimold, A.M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Karras, J.G., Wang, Z., Coniglio, S.J., Frank, D.A. & Rothstein, T.L. Antigen-receptor engagement in B cells induces nuclear expression of STAT5 and STAT6 proteins that bind and transactivate an IFN-γ activation site. J. Immunol. 157, 39–47 (1996).
Dent, A.L., Shaffer, A.L., Yu, X., Allman, D. & Staudt, L.M. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276, 589–592 (1997).
Ye, B.H. et al. The bcl-6 proto-oncogene controls germinal-centre formation and TH2- type inflammation. Nat. Genet. 16, 161–170 (1997).
Fukuda, T. et al. Disruption of the bcl-6 gene results in an impaired germinal center formation. J. Exp. Med. 186, 439–448 (1997).
Toyama, H. et al. Memory B cells without somatic hypermutation are generated from bcl-6-deficient B cells. Immunity 17, 329–339 (2002).
Ariyoshi, K. et al. Constitutive activation of STAT5 by a point mutation in the SH2 domain. J. Biol. Chem. 275, 24407–24413 (2000).
Heemskerk, M.H. et al. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J. Exp. Med. 186, 1597–1602 (1997).
Brummelkamp, T.R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Vyth-Dreese, F.A., Dellemijn, T.A., Majoor, D. & de Jong, D. Localization in situ of the co-stimulatory molecules B7.1, B7.2, CD40 and their ligands in normal human lymphoid tissue. Eur. J. Immunol. 25, 3023–3029 (1995).
Guikema, J.E. et al. Multiple myeloma related cells in patients undergoing autologous peripheral blood stem cell transplantation. Br. J. Haematol. 104, 748–754 (1999).
Acknowledgements
We thank A. Bakker (Netherlands Cancer Institute, Amsterdam, Netherlands) for help in cloning; B. Hooibrink (Academic Medical Center, Amsterdam, Netherlands), A. Pfauth and F. van Diepen (Netherlands Cancer Institute, Amsterdam, Netherlands) for help with cell sorting; T. Dellemijn, L. Oomen and L. Brooks (Netherlands Cancer Institute, Amsterdam, Netherlands) for help with CLSM analyses of the tonsil sections and transduced B cell samples; N. van der Stoep (Leiden University Medical Center, Leiden, Netherlands) for help with the luciferase assays; and the Department of Otolaryngology, Academic Medical Center, Amsterdam, Netherlands (W. Fokkens) for providing tonsil tissue.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A patent has been filed (application PCT/5B2002/005753) based on the findings presented here and a company has been founded to commercialize the findings reported here.
Supplementary information
Supplementary Fig. 1
Flow cytometric analysis of a representative culture of CA-STAT5b-ER-IRES-ΔNGFR transduced B cells cultured in CD40L, IL-2 and IL-4 in the presence of 4HT. (PDF 219 kb)
Supplementary Fig. 2
Expression of WT-STAT5b-ER in IgG+ cells results in a 4HT dependent expansion in the presence of CD40L, IL-2 and IL-4. (PDF 83 kb)
Supplementary Fig. 3
Effect of BCL6 knock down on the growth of CA-STAT5b transduced B cells. (PDF 88 kb)
Supplementary Fig. 4
Activation of CA-STAT5-ER by 4HT upregulates MYC in transduced primary CD19+ cells. (PDF 106 kb)
Supplementary Fig. 5
Activation of CA-STAT5-ER by 4HT upregulates PAX5 in transduced primary CD19+ cells. (PDF 137 kb)
Rights and permissions
About this article
Cite this article
Scheeren, F., Naspetti, M., Diehl, S. et al. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol 6, 303–313 (2005). https://doi.org/10.1038/ni1172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1172
This article is cited by
-
Signal transducer and activator of transcription (STAT)-5: an opportunity for drug development in oncohematology
Oncogene (2019)
-
Implications of STAT3 and STAT5 signaling on gene regulation and chromatin remodeling in hematopoietic cancer
Leukemia (2018)
-
IL-21 acts as a promising therapeutic target in systemic lupus erythematosus by regulating plasma cell differentiation
Cellular & Molecular Immunology (2015)
-
Novel CTCF binding at a site in exon1A of BCL6 is associated with active histone marks and a transcriptionally active locus
Oncogene (2015)
-
B cell Biology: An Overview
Current Allergy and Asthma Reports (2014)