Abstract
By convention, presentation of major histocompatibility complex (MHC) class I–restricted epitopes involves processing by cytosolic proteasomes, whereas MHC class II–restricted epitopes are generated by endosomal proteases. Here, we show that two MHC class II–restricted epitopes within influenza virus were generated by a proteasome- and TAP-dependent pathway that was accessed by exogenous virus in dendritic cells (DCs) but not cell types with less permeable endosomes. Both epitopes were presented by recycling MHC class II molecules. Challenging mice with influenza or vaccinia viruses demonstrated that a substantial portion of the MHC class II–restricted response was directed against proteasome-dependent epitopes. By complementing endosomal activities, this pathway broadens the array of MHC class II–restricted epitopes available for CD4+ T cell activation.
Similar content being viewed by others
Main
T lymphocytes are crucial for adaptive immune responses that are initiated upon recognition of peptides displayed by MHC class I or II molecules at the surfaces of antigen-presenting cells (APCs). Typically, the MHC class I processing pathway for most antigens initiates in the cytosol, the site of primary fragmentation by the multicatalytic proteasome and perhaps other proteases1,2. The resultant peptides are conveyed by the transporter of antigenic peptides heterodimer (TAP) to the endoplasmic reticulum (ER)3, where they can undergo amino terminal trimming before being loaded onto nascent MHC class I–β2-microglobulin heterodimers. These peptide-MHC complexes transit through the constitutive secretory pathway to the plasma membrane. The closed nature of the MHC class I peptide-binding groove dictates that the final length of most presented peptides be 8–11 amino acids. In contrast, the conventional MHC class II processing pathway features fragmentation and loading of internalized (exogenous) antigens within the endocytic compartment. Nascent MHC class II αβ heterodimers are guided to that location by coassembly with invariant chain (Ii), which contains a sorting signal within its cytosolic tail4,5. Within the late endosome, Ii is digested to leave only the portion that occupies the peptide-binding groove. This fragment, termed CLIP, is subsequently exchanged in the late endosome for fragmented antigens, a process mediated by the H2-M chaperonin6,7,8. Because the MHC class II peptide-binding groove is open at either end, processing is essentially a matter of antigen unfolding rather than fragmentation to a specific length. The final 11–17-amino-acid length of most MHC class II–restricted peptides loaded in late endosomes may be established by endocytic exoproteases after binding of the core epitope to MHC class II9,10. An important but largely overlooked alternative to the late endocytic pathway involves antigens that naturally unfold in the early endosome, are susceptible to the limited proteolysis in that compartment, or both. In such cases, epitopes can be captured by 'recycling' MHC class II molecules, without a requirement for Ii and H2-M expression11,12,13.
Several key exceptions to these conventional pathways have come to light. For example, the presentation of some MHC class I–restricted antigens can be independent of proteasomes and of TAP, with processing and loading occurring in the endosomal compartment14,15. Alternatively, exogenous antigen can be delivered to the conventional MHC class I processing pathway in certain cell types, such as dendritic cells (DCs) and a subset of macrophages. These cells naturally allow for transport of endosomal contents to the cytosol, and subsequently processed antigen is directed by TAP to either the ER or the endosomal compartment for loading onto MHC class I molecules16,17,18,19,20. This pathway provides a means for MHC class I–restricted cross-priming whereby antigen expressed in one cell type (owing to, for example, viral tropism) is transferred to a 'professional' APC, most likely the dendritic cell, that expresses the co-stimulatory molecules that are essential for T cell activation21. Finally, earlier work points to an endogenous pathway for MHC class II–restricted presentation that preferentially accesses antigen that has been synthesized within the cell, the standard picture for MHC class I– but not MHC class II–restricted presentation22,23,24. Although such a pathway may have an important role in responses to viral infections and transformed cells, its basis remains poorly understood.
Here we report a cytosolic (endogenous) MHC class II–restricted processing pathway elucidated through the study of three epitopes within A/PR/8/34 (PR8) influenza, all restricted to the same MHC class II molecule, I-Ed. Two epitopes reside in the hemagglutinin (HA) glycoprotein, amino acids (aa) 107–119 (S1) and aa 302–313 (S3). The third resides within the neuraminidase (NA) glycoprotein, aa 79–93 (NA79). We sought the basis for a longstanding observation22 that ultraviolet light (UV)-inactivation of the virus has little impact on S1 presentation, while strongly diminishing and ablating S3 and NA79 presentation, respectively. We show that the latter two epitopes were efficiently generated through a pathway that involves participation of the proteasome, TAP and recycling MHC class II molecules.
Results
Presentation of virus by B cells versus DCs
As reported earlier22 and shown here (Fig. 1), when mouse A20 B cell lymphoma cells were used as APCs, UV inactivation of PR8 minimally affected the presentation of HA-derived S1, but substantially diminished and essentially eliminated presentation of S3 and NA-derived NA79, respectively. Primary activated mouse splenic B cells and L929 fibroblasts stably transfected with the I-Ed α and β genes (L-I-Ed cells) showed similar presentation phenotypes (Fig. 1). The conventional model of MHC class II–restricted presentation, involving endosomal processing and loading of epitope, is difficult to reconcile with the selective presentation of S3 and NA79 from endogenous sources of HA and NA. After synthesis, neither protein is internalized to a substantial degree from the default destination of the plasma membrane, the requisite location for viral assembly. Even if HA and NA were internalized to a meaningful extent or if antigen were transferred (cross-presented) to other cells, it is not obvious how processing would differ from that of virion-associated HA and NA. Evidence is also lacking for the direct trafficking of nascent HA and NA to later endosomal compartments that might be more conducive to the generation of MHC class II epitopes such as S3 and NA79.
The indicated APCs (5 × 104) were pulsed with infectious PR8 (▪), UV inactivated PR8 (▴) or infectious, antigenically distinct B/Lee (▾) at the indicated doses. APCs were cocultured with 1 × 105 zS1.1 or zNA79.1 or 1.5 × 105 zS3.1 T cell hybridomas for 18 h. β-galactosidase production after pMHC recognition and subsequent activation of the T cell hybrids was measured using X-gal substrate. These experiments were carried out at least four times and the results of a representative experiment are shown here. Error bars represent the s.e.m. of experimental replicates.
The possibility of an alternative processing pathway was suggested when primary immature bone marrow–derived dendritic cells (DCs) were used as APCs. In this case, all three epitopes were presented with equally high efficiency from inactivated and live virus (Fig. 1). Although this result could be explained by a fundamentally distinct composition of DC endosomal compartments, we considered an alternative possibility based on two previous observations. First, it has been demonstrated that DCs readily deliver their endosomal contents to the cytosol for proteasome-dependent MHC class I–restricted processing19. Second, earlier work has implicated the proteasome in the MHC class II–restricted presentation of certain endogenously expressed cytosolic antigens25,26. Thus, if the generation of NA79 epitope were entirely dependent on the proteasome, then retrograde translocation of nascent NA from the ER to the cytosol would provide the only means of presentation by cells with relatively impermeable endosomes. This is a scheme thus far associated only with MHC class I–restricted antigen processing27. This would not be the case with DCs, which could deliver endocytosed UV-inactivated influenza virus to the cytosol. In contrast, if S1 epitope presentation were entirely dependent on endosomal processing, the APC type and UV-inactivation of the virus would have relatively little impact on presentation. Presentation of the S3 epitope could be explained if it were generated by either pathway.
Cytosolic delivery of exogenous influenza by DCs
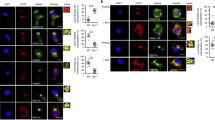
We investigated the natural delivery of input virus to the cytosol of DCs in two ways. First we traced the fate of fluorescein isothiocyanate (FITC)-labeled virions pulsed into DCs and L-I-Ed cells (Fig. 2a). A predominantly punctate staining pattern, indicative of retention in the endosomal compartment, was observed in both cell types at 1 h after the pulse, and this was maintained in L-I-Ed cells at the 6-h mark. In contrast, by 6 h after the pulse, the pattern in DCs was diffuse and homogeneous, consistent with a cytosolic pattern. Second, we investigated the ability of B cells and DCs to deliver exogenous UV-inactivated virus to the classical MHC class I–restricted pathway, by assessing presentation of a proteasome- and TAP-dependent epitope within influenza nucleoprotein (NP366–374) that is restricted by H2-Db (ref. 28). Although both primary B cells and DCs were able to present NP366–374 from infectious virus, only DCs generated the epitope from UV-inactivated virus (Fig. 2b). To confirm that differences in presentation capabilities were due to the relative ease with which endocytosed virus was transferred to the cytosol in DCs, we provoked cytosolic delivery through osmotic rupture of virion-bearing endosomes29 in A20 cells, which, like L-I-Ed cells, do not present the NA79 epitope from exogenous sources of virus (Fig. 1). This procedure substantially enhanced presentation of S3 and NA79 from UV-inactivated virus (Fig. 2c). Thus, delivery of exogenous influenza to the cytosol, which occurs naturally in DCs, was crucial for the efficient MHC class II–restricted presentation of the S3 and NA79 epitopes. This is the expected situation, as shown here, for MHC class I– but not MHC class II–restricted antigen processing.
(a) L-I-Ed or primary immature DCs were pulsed with 500 HAU of FITC-labeled PR8 virus (FITC-PR8), chased for the indicated times and imaged by fluorescence microscopy. Two independent experiments were done with similar results. (b) Indicated APCs (1 × 106 cells) from C57BL/6 mice were pulsed with 100 HAU of infectious B/Lee, PR8 (iPR8) or UV-inactivated PR8 (uvPR8) per 1 × 106 cells, double diluted (starting at 2 × 105 cells/well), and cocultured with 1.5 × 105 CD8+ T cell hybrids specific for NP366–374/Db. Two independent experiments were carried out with similar results. Error bars represent the s.e.m. of experimental replicates. (c) A20 cells (106) were incubated with 100 HAU of inactivated PR8 or B/Lee in hypertonic medium with or without PEG. Cell suspensions were briefly exposed to hypotonic medium, washed, double diluted (starting at 105 cells/well) and cocultured with epitope-specific T cell hybrids. Presentation of S1, S3 and NA79 epitopes was subsequently measured. Background values obtained with B/Lee were subtracted from PR8 values. Two independent experiments were carried out with similar results. Error bars represent the s.e.m. of experimental replicates.
Proteasome-dependent presentation
The importance of cytosolic delivery for enhanced presentation of the S3 epitope and any presentation of the NA79 epitope is consistent with the hypothesis, outlined above, that the proteasome, located in the cytosol and nucleus, is involved in the presentation of these two epitopes. To test this directly, we treated primary B cells (Fig. 3a) and A20 cells (data not shown) with epoxomicin, a highly specific proteasome inhibitor30. This compound had minimal impact on the presentation of any epitope from UV-inactivated virus. This was expected, because presentation under these conditions would be limited to endocytic compartments that are devoid of proteasomes. Using the same cells, we then tested the impact of the inhibitor with a dose of live virus that limits the presentation of S3 from input virions, thus emphasizing endogenous presentation. Under these conditions, which allow for cytosolic delivery of the antigens as a result of the retrograde translocation mechanism discussed above, epoxomicin completely inhibited presentation of the S3 and NA79 epitopes. Presentation of the S1 epitope was unaffected (Fig. 3a). As the S3 epitope is also generated through endocytic processing (Fig. 1), one would predict that a high dose of live virus, providing sufficient input virions, will limit the impact of proteasome inhibitor. Indeed, this was what we observed (Supplementary Fig. 1 online). When DCs were used as APCs, epoxomicin treatment profoundly inhibited presentation of the NA79 and S3 epitopes from both UV-inactivated and infectious virus, whereas S1 epitope presentation was unperturbed (Fig. 3a). This was also predictable given the cytosolic delivery of exogenously provided virus in DCs. Epoxomicin treatment had no effect either on the presentation of synthetic peptides (Fig. 3a) or on the abundance of surface MHC class II (data not shown). Notably, when A20 cells were treated with another proteasome inhibitor, lactacystin, S3 epitope presentation from inactivated virus was inhibited (Supplementary Fig. 2 online). However, lactacystin has recently been reported to inhibit cathepsin A, an endosomal protease31, indicating a specificity that goes beyond the proteasome. We have previously proposed that a proteolytic step is involved in the endocytic processing of the S3 epitope from exogenous virus to allow for release of a S3-containing fragment from the virion32. Perhaps lactacystin interferes with this release step, although it is unlikely to be mediated by cathepsin A, a carboxypeptidase. Together, these results strongly support a role for the proteasome in the generation of the MHC class II–restricted S3 and NA79 epitopes but not of the classical S1 epitope.
(a,b) Indicated APCs (106) were pretreated with the proteasome inhibitor epoxomicin (epox) (a) or the thiol protease inhibitor leupeptin (leup) (b), or left untreated. APCs were pulsed with a low dose (4 HAU) of infectious PR8 (iPR8) or B/Lee, a high dose (100 HAU) of UV-inactivated PR8 (uvPR8) or B/Lee, or synthetic peptides in the presence or absence of inhibitor. APCs were double diluted (starting at 1 × 105 cells/well) and cocultured in duplicate with zS1.1, zS3.1 or zNA79.1 T cell hybrids. Single samples were then used for peptide controls within the epoxomicin experiment. T cell hybrid responses were then measured using X-gal substrate. Background values obtained with B/Lee were subtracted from the values obtained with PR8. Error bars represent the s.e.m. of experimental replicates. Data are a representative example of three independent experiments.
In a complementary approach, we assessed the effect of leupeptin, an inhibitor of endosomal thiol proteases, on epitope presentation. As observed previously33 and shown here (Fig. 3b), when A20 cells were pulsed with inactivated virus, leupeptin inhibited the presentation of S1 while considerably enhancing the presentation of S3. This latter effect is due to the acid-mediated unfolding of HA shortly after virus uptake, a step that allows for loading of the S3 epitope onto recycling MHC class II molecules but also renders the epitope highly susceptible to proteolysis13,34. In contrast, when infectious virus was presented by A20 cells or DCs, or when inactivated virus was presented by DCs, leupeptin had no effect on S3 presentation but continued to inhibit S1 presentation (Fig. 3b). NA79 epitope presentation was unaffected by leupeptin (data not shown) and it remains to be seen whether the failure of this epitope to be presented through the conventional pathway is due to an inability of the endosomal machinery to produce the epitope, to destruction of the NA79 epitope by leupeptin-insensitive endosomal protease(s) or both. At the concentrations used, epoxomicin and leupeptin did not affect MHC class II expression or viral uptake as determined by immunofluorescence studies (data not shown). Thus, cytosolic processing of the S3 epitope is functionally distinct from endocytic processing, in that it is proteasome dependent and leupeptin insensitive.
TAP and recycling MHC class II molecules
Previous work has described an MHC class I processing pathway for DCs and a macrophage subset that involves TAP-mediated delivery of proteasome-dependent epitopes to a fused ER-phagosome vesicle where empty MHC class I molecules reside19,20. To determine whether a TAP-dependent scheme might be involved in the presentation of influenza epitopes S3 and NA79, we transduced fibroblast lines from Tap1−/− and Tap1+/+ mice with retroviruses that express the I-Ed α and β chains32 and tested them as APCs. Although Tap1+/+ fibroblasts presented all three epitopes efficiently, the Tap1−/− fibroblasts were completely unable to present S3 and NA79 from infectious PR8, whereas their presentation of S1 was essentially the same as for Tap1+/+ fibroblasts (Fig. 4a). Fibroblasts from Tap1−/− and Tap1+/+ mice presented synthetic peptides to a similar extent, indicating that I-Ed was expressed equally in both sets of APCs (Fig. 4a). We took a similar approach with primary DCs from Tap1−/− and Tap1+/+ mice. Transduction was much less efficient with DCs and T cell activation was, therefore, much lower than with the fibroblast lines. Nevertheless, comparison of untransduced and transduced pairs indicated that DC presentation of epitopes S3 and NA79, but not S1, was TAP dependent (Fig. 4b). Consistent with cytosolic delivery of exogenous virus by DCs, presentation of S3 and NA79 from UV-inactivated virus was also TAP dependent.
(a) Primary fibroblasts from a Tap1+/+ mouse and a Tap1−/− mouse were transduced with retroviruses expressing H2-I-Ed α chain, β chain and hCIITA (αβ) or hCIITA alone (CIITA). These two populations were then pulsed with 250 HAU of infectious PR8 (iPR8) per 106 cells or synthetic peptides. (b) Primary DCs from a Tap1+/+ mouse and Tap1−/− mouse were untreated (no RV) or transduced with retroviruses expressing H2-I-Ed α chain and β chain (αβ). These two populations were then pulsed with 250 HAU of infectious PR8 (iPR8) or UV-inactivated PR8 (uvPR8) per 106 cells or synthetic peptides. In both a and b, retrovirally transduced APCs were double diluted (starting at 5 × 104 cells/well) and cocultured with zS1.1, zS3.1 and zNA79.1 T cell hybrids. T hybrid responses were then measured using X-gal substrate. Because of limited numbers of primary fibroblasts and DCs, most APC/T hybrid ratios were tested with single samples. Error bars for NA79-specific responses in b indicate the s.e.m. of experimental replicates. Two independent experiments were done with similar results.
Our previously published experiments13,34 indicate that the S3 epitope is generated from exogenous sources in the early endosome and loads onto recycling MHC class II molecules in that compartment without the assistance of the peptide-exchanging chaperonin H2-M. In contrast, S1 is generated in a late endosomal compartment and loads onto nascent I-Ed in an H2-M-dependent manner. Using wild-type A20 cells and A20 cells deficient in H2-M (A20-3A5)35, we observed that presentation of proteasome-dependent epitopes S3 and NA79 also proceeded in the absence of H2-M, whereas S1 was absolutely dependent on H2-M (Supplementary Fig. 3 online). To determine whether the proteasome-dependent presentation of S3 and NA79 epitopes, like that of endocytically processed S3, depended on recycling MHC class II molecules, we investigated the effect of primaquine on their presentation. This compound blocks transit of MHC class II molecules from the early endosome to the cell surface, thereby inhibiting presentation by recycling MHC class II molecules13. Exposure of DCs to primaquine had no effect on S1 presentation but severely inhibited presentation of epitopes S3 and NA79 from both UV-inactivated and infectious virus (Fig. 5). Primaquine treatment did not have an impact on MHC class II expression or viral uptake as determined by immunofluorescence studies (data not shown). Together, the data indicated that proteasome-dependent NA79 and S3 epitopes may require TAP for delivery to the early endosome, where they loaded onto recycling MHC class II molecules, as is the case for endosomally produced S3. Given the open-ended nature of the MHC class II peptide-binding groove, additional processing of the cytosolically processed antigens in the endosome may be unnecessary.
DCs were pretreated with primaquine (primaq), an inhibitor of recycling MHC class II molecules, or left untreated. APCs were pulsed with a low dose (4 HAU) of infectious PR8 (iPR8) or B/Lee, a high dose (100 HAU) of UV-inactivated PR8 (uvPR8) or B/Lee, or synthetic peptides in the presence or absence of inhibitor. APCs were double diluted (starting at 5 × 104 cells/well) and cocultured with zS1.1, zS3.1 and zNA79.1 T cell hybrids. T cell hybrid responses were then measured using X-gal substrate. Background values obtained with B/Lee were subtracted from PR8 values. This experiment was carried out several times, and the results of a representative experiment are shown here. Error bars represent the s.e.m. of experimental replicates.
In vivo responses to proteasome-dependent epitopes
To gain a better appreciation for the frequency of proteasome-dependent epitopes and their impact on in vivo responses, we immunized mice with live PR8 virus. Four weeks later, we used an interferon-γ-based ELISPOT assay to test splenocytes for reactivity to L-I-Ed cells that were pulsed with infectious or UV-inactivated PR8 in the presence or absence of epoxomicin. Because L929 cells are H2k, only I-Ed-restricted influenza-specific T cells will respond. This strategy prevented MHC class I–restricted viral responses, which we expected to be substantially diminished by proteasome inhibitor, from confounding the analysis. An appreciable fraction of the response (∼30–40%), with each spot indicating an influenza-specific CD4+ T cell, was lost when the proteasome inhibitor was used (Fig. 6a). As predicted from the results discussed above (Fig. 3a), presentation of UV-inactivated virus was not influenced significantly by epoxomicin treatment, because these conditions limited processing to the endocytic compartment. We validated this approach to analyzing populations developed in vivo by immunizing mice with synthetic S1 or NA79 epitope peptides and subjecting the responding CD4+ T cells to the same ELISPOT assay. Consistent with the data shown above, the ELISPOT response of NA79-specific T cells was profoundly diminished by addition of proteasome inhibitor, whereas the response of S1-specific T cells was not (Fig. 6a, inset). We also analyzed the relative numbers of S1-, S3- and NA79-specific CD4+ T cells that develop after influenza infection by stimulation of immune splenocytes with peptide-pulsed L-I-Ed cells in an ELISPOT assay. The results showed that the responses to S1, S3 and NA79 after viral infection were comparable in magnitude (Supplementary Fig. 4 online), indicating that proteasome-dependent epitopes elicited substantial responses in vivo. Finally, we analyzed the MHC class II–restricted response to vaccinia virus by ELISPOT assay to determine whether the proteasome-dependent CD4+ T cell response to another virus was similar in scope. Mice were inoculated with the conventional WR strain of vaccinia and the immune splenocytes were stimulated with L-I-Ed cells infected with modified vaccinia Ankara (MVA). The use of MVA, which does not complete replication in most mammalian cells but is highly homologous with WR, eliminated the strong cytopathic effects of vaccinia that complicate the ELISPOT assay. As we observed with the influenza-specific response, the numbers of vaccinia-specific CD4+ T cells were considerably reduced by proteasome inhibitor, whereas those to UV-inactivated virus were not (Fig. 6b). Together, these data indicated that substantial portions of CD4+ T cell responses to the viral infections might be specific for proteasome-dependent epitopes.
(a,b) L-I-Ed cells (106) were first pretreated with epoxomicin (epox) or left untreated; next they were infected with 100 HAU of infectious B/Lee or PR8 (iPR8) or were pulsed with 100 HAU of UV-inactivated B/Lee or PR8 (UVPR8; a), or they were pulsed with 5 × 105 PFU infectious MVA (MVA) or UV-inactivated MVA (UVMVA) or were left untreated (b). These L-I-Ed cells (2 × 105 per well) were used to re-stimulate splenocytes from (a) iPR8- or (b) vaccinia-immunized BALB/c mice in a standard IFN-γ-based ELISPOT assay. In a, background values obtained with B/Lee were subtracted from PR8 values. Inset in a, L-I-Ed cells (1 × 106) were pretreated with epoxomicin (epox) or left untreated and infected with 100 HAU infectious B/Lee or PR8 (iPR8). In addition, untreated L-I-Ed cells were pulsed with S1 or NA79 synthetic peptides. These APCs were used as stimulators and incubated with lymph node cells from either S1- or NA79-peptide-immunized mice in an IFN-γ ELISPOT assay. Background values obtained from mice immunized with CFA alone were subtracted from peptide and PR8 values. Three independent PR8 and MVA experiments and two independent peptide experiments were carried out and the results of a representative experiment are presented here. Error bars represent the s.e.m. of experimental replicates.
Discussion
These findings further blur the distinctions between MHC class I– and MHC class II–restricted antigen processing and presentation, and reinforce the notion that the immune system uses all means possible to identify foreign entities. We speculate that the mutual existence of the endocytic and cytosolic MHC class II–restricted presentation pathways is due to nonredundant processing capabilities of these compartments. The endocytic system is unable to generate epitopes such as NA79, whereas the cytosolic system is unable to generate epitopes such as S1. Because the key step in MHC class II–restricted processing is unfolding of the antigen that can be accomplished in either compartment, the explanation for such partitioned processing may not be the lack of certain proteases in the processing-incompetent compartment. Rather, it may be the presence of certain proteases that destroy the epitope before sufficient amounts can be delivered to receptive MHC class II molecules. This idea is supported by the case of endosomally generated S3, which barely survives endosomal proteolysis, as the effects of leupeptin demonstrate. The NA79 epitope may be even more susceptible to endosomal proteases, whereas the S1 epitope may be sensitive to cytosolic proteolysis.
It remains to be seen whether there is a bias toward presentation of proteasome-dependent epitopes by recycling MHC class II molecules. We have previously emphasized the importance of recycling MHC class II molecules for the presentation of endosomally generated epitopes such as S3 that are revealed in the early endosome13. The presentation of proteasome-dependent epitopes may provide another reason for their existence. Owing to the involvement of recycling MHC class II molecules and the lack of ER-phagosomes in B cells and fibroblasts, we favor the notion of TAP-mediated delivery to the early endosome. Another intriguing possibility is that aspects of S1-like epitopes that allow for presentation by the conventional pathway preclude presentation through the cytosolic pathway. Additional work will be required to better define these and other aspects of the proteasome- and MHC class II–dependent processing pathway.
It is readily apparent how this pathway, and the epitopes that emerge from it, would be unappreciated in many conventional systems. The foreign proteins that are commonly used for studies of MHC class II responses generally elicit poor MHC class I–restricted responses, presumably because the immunogens do not enter the cytosol of DCs with great efficiency in vivo. It follows that the same is true for proteasome-dependent MHC class II–restricted epitopes. In contrast, the priming of robust MHC class I responses by immunization with infectious virus, through cross-presentation, has been well documented36,37,38. Additionally, if in vitro assays use proteins, cell lysates or inactivated organisms in conjunction with APCs other than DCs, T cells specific for proteasome-dependent epitopes would not be detected even if they were present in the responder population because only endosomally generated epitopes would be displayed.
It is likely that DCs acquire the components of many agents through cross-presentation from infected cells39. Thus, given the key role of DCs in the initiation of T cell responses, it would appear mandatory that they possess a mechanism for delivery of exogenous material to the proteasome in order for the elicited CD4+ T cell population to be specific for the full range of epitopes that are expressed, perhaps disparately, at the site or sites of infection. At such sites, some cells will be directly infected, emphasizing the cytosolic pathway, whereas others will acquire exogenous antigens that are released by infected cells, emphasizing the endosomal pathway. Clinical evidence suggests the importance of broadly specific CD8+ and CD4+ T cell responses for the containment of certain viral infections40,41. Thus, accounting for the proteasome-dependent pathway may be an important component in vaccine design.
Methods
Mice.
Six-week-old female BALB/c (H-2d) and C57BL/6 (H-2b) mice, obtained from Taconic Farms, and C57BL/6 Tap1−/− (H-2b) mice, received from NIAID/Taconic Repository, were maintained by the Thomas Jefferson University Office of Laboratory Animal Services (Philadelphia, Pennsylvania, USA). All experimental protocols were preapproved by the Thomas Jefferson University Institutional Animal Care and Use Committee (IACUC).
Primary APCs and APC cell lines.
Immature dendritic cells were generated as described previously42. Briefly, bone marrow precursor cells were harvested and grown in RPMI 1640 (Fisher Scientific) supplemented with 5% FBS (HyClone), 0.05 mM 2-mercaptoethanol (2-ME; Sigma-Aldrich) and 10 ng/ml mouse recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) (PeproTech). Cells were left undisturbed for 6 d at 37 °C in 6% CO2. On day 6, semiadherent cells were harvested, washed with PBS and used as APCs, which were phenotyped for immature DC markers (cd11b+, cd11c+, CD80− and CD86− status) by flow cytometry. Small, dense B cells were isolated from spleens by positive selection on a MoFlo machine (Dako Cytomation) using R-phycoerythrin (PE)-conjugated monoclonal anti–mouse B220 (Pharmingen). Sorted B cells were then incubated in vitro in RPMI 1640 with 10% FBS and activated with 10 μg/ml LPS (Sigma-Aldrich) for 48 h. Activation of B cells was determined by upregulation of MHC class II, CD80 and CD86. Primary fibroblasts from Tap1+/+ (wild-type) and Tap1−/− (knockout) mice were generated from skin biopsies according to standard procedures. Both A20.2J, an I-Ed positive B lymphoma43, and A20 3A5, a B lymphoma defective in H2-DMα chain44, were maintained in RPMI supplemented with 5% FBS (Sigma) and 0.05 mM 2-ME. L-I-Ed cells45, derived from the parent clone L929 and transfected with I-Ed, were maintained in IMDM supplemented with 5% FBS and hypoxanthine, aminopterin and thymine (HAT) (Sigma-Aldrich).
T cell hybridomas.
S1- and S3-specific T cell hybridomas (zS1.1 and zS3.1, respectively), which express β-galactosidase upon recognition of peptide–MHC class II complexes, and NP366–374-specific T hybridoma (DBFZ.25), which express β-galactosidase upon recognition of peptide–MHC class I complexes, have been described previously13,46. T cell hybridomas were maintained in RPMI supplemented with 10% FBS and 0.05 mM 2-ME. NA79-specific T cell hybridomas (zNA79.1) that express β-galactosidase upon activation were generated by fusion of an NA79-specific CD4+ cytotoxic T cell clone47 with the fusion partner BWZ.3646. The specificity of this T cell hybridoma is demonstrated in Supplementary Figure 5 online.
Influenza viruses.
Influenza viruses, A/Puerto Rico/8/34 (PR8), subtype H1N1, and viruses antigenically distinct from PR8, B/Lee/40 (B/Lee), lacking the S1, S3, NA79 and NP epitopes, and A/Japan/305+/57 (JAP), lacking the S1, S3 and NA79 epitopes, were grown in the allantoic cavity of 10-d embryonated chicken eggs and harvested on day 12. Viral titers were determined by hemagglutination of chicken erythrocytes. Infectious virus was UV-inactivated by exposure to short-wave (254-nm) light (Stratalinker 1800, Stratagene) for 1 min (30-s intervals) in 1-ml volumes in 60 × 15–mm Petri dishes (Fisher). Mouse fibroblasts pulsed with UV-inactivated virus showed no de novo synthesis of influenza nucleoprotein as assessed by immunohistochemistry. PR8 virus was purified as described previously34 and labeled using fluorescein isothiocyanate (FITC) on Celite (Sigma-Aldrich). Purified PR8 was incubated with FITC in PBS for 8 h at 4 °C. Labeled virus was separated from free FITC by PBS dialysis and viral integrity and titer were measured by hemagglutination.
Synthetic peptides.
Synthetic S1 (aa 107–119), S3 (aa 302–313) and NA79 (aa 79–93) peptides were purchased from Invitrogen and reconstituted in dimethyl sulfoxide (DMSO).
Antigen presentation assays and inhibitory compounds.
APCs were pulsed with either inactivated or infectious PR8 or B/Lee viruses at indicated doses for 1 h at 37 °C in a tube rotator. APCs were then washed and cocultured with T cell hybridomas for 18–20 h and β-galactosidase production by activated T hybrids was measured using X-gal as the chromogenic substrate as described previously46. The following compounds were used at the indicated concentrations: epoxomicin (Boston Biochem) at a final concentration of 20 μM; leupeptin (Sigma) at 0.5 mM; and primaquine (Aldrich) at 100 μM. In presentation assays using inhibitory compounds, APCs were pretreated with the indicated compounds 1 h before viral pulse and then were pulsed with the indicated viruses in the presence of the inhibitors. APCs were then washed and were cocultured in the presence (for leupeptin or primaquine) or absence (for epoxomicin) of the inhibitor with T cell hybridomas, and T hybrid activation was measured as described above.
Osmotic lysis of endosomes.
This method was as previously described48. Briefly, inactivated viruses were resuspended in hypertonic medium containing 10% (wt/vol) polyethylene glycol (PEG) 400 (Sigma) or hypertonic medium alone and added to the A20 cell pellet and incubated for 10 min at 37 °C. Cell suspensions were then diluted with prewarmed hypotonic medium for 2–3 min at 37 °C. Cells were pelleted and co-incubated with T hybrids and a presentation assay was carried out as described above.
Immunofluorescence microscopy.
Immature DCs and L-I-Ed cells were pulsed with FITC-labeled or mock-treated PR8 on ice for 30 min, washed three times with PBS, and chased for indicated times at 37 °C. APCs were plated on coverslips before fixation with 2% paraformaldehyde. Cells were mounted in Mowiol (Sigma-Aldrich) and analyzed with a Zeiss AxioVert 200M microscope (Carl Zeiss) at the Kimmel Cancer Center Bioimaging Facility.
Recombinant retroviruses and transduction of cells.
Recombinant retroviruses expressing H2-Ed α and β chains and hCIITA were generated and have been described previously32. Cell transductions of primary skin fibroblasts were carried out as described earlier32. Briefly, primary skin fibroblasts were transduced with the indicated recombinant retroviruses in the presence of polybrene (10 μg/ml) (Sigma) for 12 h. Cells were then washed and cultured in DMEM supplemented with 10% FBS (HyClone) for an additional 48 h before being used in antigen presentation assays. Retroviral transduction of primary DCs was carried out as described earlier49. Briefly, before DC differentiation, on day 2, precursor cells in culture were transduced with the recombinant retroviruses in the presence of polybrene (8 μg/ml) and HEPES (10 mM) and spun in a tabletop centrifuge at 32 °C, 1,083g for 2 h. After centrifugation, retroviruses were aspirated and cells were fed fresh DC growth medium (see above) and cultured for an additional 48 h after infection. DCs were then harvested, washed and used in antigen presentation assays.
Immunizations and ELISPOT assays.
Six-week-old female BALB/c (H-2d) mice were immunized by i.p. injection with 100 hemagglutinating units (HAU) infectious PR8 or with vaccinia virus (107 plaque-forming units (PFU)) or phosphate-buffered saline solution alone. To measure the S1- and NA79-epitope-specific responses, immunizations were carried out as described previously50. Briefly, 6-week-old female BALB/c (H-2d) were immunized with 50 μg of S1, NA79 or no peptide emulsified in complete Freund's adjuvant (CFA) (DIFCO) in one hind footpad. After 10 d, the draining popliteal lymph nodes were removed, and cell suspensions were prepared and used in ELISPOT assays. For ELISPOT assays, L-I-Ed cells were (i) pretreated for 15 min with epoxomicin or left untreated, and then (ii) for 45 min in the presence or absence of epoxomicin, either pulsed or infected with UV-inactivated or live B/Lee or PR8 viruses, or infected or pulsed with live modified Vaccinia Ankara (MVA) virus, UV-inactivated MVA or no viruses. APCs were then washed and cultured for 7 h in IMDM supplemented with 5% FBS. Additionally, untreated or epoxomicin-treated L-I-Ed cells were pulsed with synthetic peptides (S1 at 100 μg/ml, S3 and NA79 at 10 μg/ml concentration) or no synthetic peptides and used as stimulators. Cells were then fixed using 0.5% paraformaldehyde for 1 min on ice, washed and used as stimulators at 2 × 105 cells per well in a 96-well ELISPOT tissue culture plate coated overnight with purified anti–IFN-γ (Pharmingen). Splenocytes from BALB/c mice immunized with infectious PR8 or vaccinia virus, or popliteal lymph nodes cells removed from peptide-immunized mice, were cocultured (5 × 105 cells per well) overnight with the L-I-Ed stimulators. The assay was developed using biotinylated anti-IFN-γ, avidin-conjugated horseradish peroxidase and 3-amino-9-ethylcarbazole (AEC) substrate (Pharmingen).
Note: Supplementary information is available on the Nature Immunology website.
References
Falk, K., Rötzschke, O. & Rammensee, H.-G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature 348, 248–251 (1990).
Rock, K.L. & Goldberg, A.L. Degradation of cell proteins and the generation of MHC class I–presented peptides. Annu. Rev. Immunol. 17, 739–779 (1999).
Heemels, M.-T. & Ploegh, H. Generation, translocation, and presentation of MHC class I–restricted peptides. Annu. Rev. Biochem. 64, 463–491 (1995).
Lamb, C.A. & Cresswell, P. Assembly and transport properties of invariant chain trimers and HLA-DR-invariant chain complexes. J. Immunol. 148, 3478–3482 (1992).
Lotteau, V. et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature 348, 600–605 (1990).
Lindstedt, R., Liljedahl, M., Péléraux, A., Peterson, P.A. & Karlsson, L. The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity 3, 561–572 (1995).
Denzin, L.K. & Cresswell, P. HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell 82, 155–165 (1995).
Kropshofer, H. et al. Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J. 15, 6144–6154 (1996).
Sette, A., Adorini, L., Colon, S.M., Buus, S. & Grey, H.M. Capacity of intact proteins to bind to MHC class II molecules. J. Immunol. 143, 1265–1267 (1989).
Sercarz, E.E. & Maverakis, E. MHC-guided processing: binding of large antigen fragments. Nat. Rev. Immunol. 3, 621–629 (2003).
Pinet, V., Vergelli, M., Martin, R., Bakke, O. & Long, E.O. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature 375, 603–606 (1995).
Lindner, R. & Unanue, E.R. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J. 15, 6910–6920 (1996).
Sinnathamby, G. & Eisenlohr, L.C. Presentation by recycling MHC class II molecules of an influenza hemagglutinin-derived epitope that is revealed in the early endosome by acidification. J. Immunol. 170, 3504–3513 (2003).
Stryhn, A. et al. pH dependence of MHC class I-restricted peptide presentation. J. Immunol. 156, 4191–4197 (1996).
Schirmbeck, R., Melber, K. & Reimann, J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur. J. Immunol. 25, 1063–1070 (1995).
Norbury, C.C., Hewlett, L.J., Prescott, A.R., Shastri, N. & Watts, C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow. Immunity 3, 783–791 (1995).
Kovacsovics-Bankowski, M. & Rock, K.L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 267, 243–246 (1995).
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1, 362–368 (1999).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003).
den Haan, J.M., Lehar, S.M. & Bevan, M.J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells In vivo. J. Exp. Med. 192, 1685–1696 (2000).
Eisenlohr, L.C. & Hackett, C.J. Class II major histocompatibility complex-restricted T cells specific for a virion structural protein that do not recognize exogenous influenza virus. J. Exp. Med. 169, 921–931 (1989).
Jaraquemada, D., Marti, M. & Long, E.O. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II restricted T cells. J. Exp. Med. 172, 947–954 (1990).
Oxenius, A. et al. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur. J. Immunol. 25, 3402–3411 (1995).
Lich, J.D., Elliott, J.F. & Blum, J.S. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J. Exp. Med. 191, 1513–1524 (2000).
Mukherjee, P. et al. Efficient presentation of both cytosolic and endogenous transmembrane protein antigens on MHC class II is dependent on cytoplasmic proteolysis. J. Immunol. 167, 2632–2641 (2001).
Wiertz, E.J.H. et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384, 432–438 (1996).
Cerundolo, V. et al. The proteasome-specific inhibitor lactacystin blocks presentation of cytotoxic T lymphocyte epitopes in human and murine cells. Eur. J. Immunol. 27, 336–341 (1997).
Okada, D.Y. & Rechsteiner, M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytotic vesicles. Cell 29, 33–41 (1982).
Schwarz, K. et al. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses. J. Immunol. 164, 6147–6157 (2000).
Ostrowska, H. et al. Separation of cathepsin A-like enzyme and the proteasome: evidence that lactacystin/β-lactone is not a specific inhibitor of the proteasome. Int. J. Biochem. Cell Biol. 32, 747–757 (2000).
Sinnathamby, G., Maric, M., Cresswell, P. & Eisenlohr, L.C. Differential requirements for endosomal reduction in the presentation of two H2-Ed-restricted epitopes from influenza hemagglutinin. J. Immunol. 172, 6607–6614 (2004).
Eisenlohr, L.C., Gerhard, W. & Hackett, C.J. Individual class II-restricted antigenic determinants of the same protein exhibit distinct kinetics of appearance and persistence on antigen-presenting cells. J. Immunol. 141, 2581–2584 (1988).
Chianese Bullock, K.A. et al. Antigen processing of two H2-IEd restricted epitopes is differentially influenced by the structural changes in a viral glycoprotein. J. Immunol. 161, 1599–1607 (1998).
Russell, H.I., York, I.A., Rock, K.L. & Monaco, J.J. Class II antigen processing defects in two H2d mouse cell lines are caused by point mutations in the H2-DMa gene. Eur. J. Immunol. 29, 905–911 (1999).
Sigal, L.J., Crotty, S., Andino, R. & Rock, K.L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398, 77–80 (1999).
Heath, W.R. et al. Cross-presentation dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199, 9–26 (2004).
Chen, W. et al. Cross-priming of CD8+ T cells by viral and tumor antigens is a robust phenomenon. Eur. J. Immunol. 34, 194–199 (2004).
Heath, W.R. & Carbone, F.R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Gandhi, R.T. & Walker, B.D. Immunologic control of HIV-1. Annu. Rev. Med. 53, 149–172 (2002).
Day, C.L. et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J. Virol. 76, 12584–12595 (2002).
Inaba, K. et al. The formation of immunogenic major histocompatibility complex class II–peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191, 927–936 (2000).
Kim, K.J., Kanellopoulos-Langevin, C., Merwin, R.M., Sachs, D.H. & Asofsky, R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 122, 549–554 (1979).
Dang, L.H., Lien, L.L., Benacerraf, B. & Rock, K.L. A mutant antigen-presenting cell defective in antigen presentation expresses class II MHC molecules with an altered conformation. J. Immunol. 150, 4206–4217 (1993).
Miller, J. & Germain, R.N. Efficient cell surface expression of class II molecules in the absence of associated invariant chain. J. Exp. Med. 164, 1478–1489 (1986).
Sanderson, S. & Shastri, N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 6, 369–376 (1994).
Hackett, C.J., Horowitz, D., Wysocka, M. & Eisenlohr, L.C. Immunogenic peptides of influenza virus subtype N1 neuraminidase identify a T-cell determinant used in class II major histocompatibility complex-restricted responses to infectious virus. J. Virol. 65, 672–676 (1991).
Moore, M.W., Carbone, F.R. & Bevan, M.J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54, 777–785 (1988).
Chow, A., Toomre, D., Garrett, W. & Mellman, I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).
Chaturvedi, P., Hengeveld, R., Zechel, M.A., Lee-Chan, E. & Singh, B. The functional role of class II-associated invariant chain peptide (CLIP) in its ability to variably modulate immune responses. Int. Immunol. 12, 757–765 (2000).
Acknowledgements
We thank S. Luke at the Kimmel Cancer Institute bioimaging facility and J. Faust and A. Acosta at the Wistar flow cytometry facility for outstanding technical assistance. This research was supported by grants from the US National Institutes of Health (AI036331) and the Leukemia and Lymphoma Society (1121-00). M.K.T. is a recipient of a National Research Service Award (T32-CA09683).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Specificity of NA79 T cell hybridomas. (PDF 87 kb)
Supplementary Fig. 2
Effect of epoxomicin on S3 presentation from increasing doses of infections PR8. (PDF 123 kb)
Supplementary Fig. 3
Presentation of S3 from exogenous virus is completely inhibited by lactacystin. (PDF 83 kb)
Supplementary Fig. 4
Role of H-2M in presentation of S1 and proteasome dependent S3 and NA70 epitopes. (PDF 85 kb)
Supplementary Fig. 5
Proteasome-dependent epitopes can generate immune responses in vivo as measured by synthetic peptides. (PDF 96 kb)
Rights and permissions
About this article
Cite this article
Tewari, M., Sinnathamby, G., Rajagopal, D. et al. A cytosolic pathway for MHC class II–restricted antigen processing that is proteasome and TAP dependent. Nat Immunol 6, 287–294 (2005). https://doi.org/10.1038/ni1171
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1171
This article is cited by
-
Cryptic MHC-E epitope from influenza elicits a potent cytolytic T cell response
Nature Immunology (2023)
-
Type II alveolar cell MHCII improves respiratory viral disease outcomes while exhibiting limited antigen presentation
Nature Communications (2021)
-
Class II MHC antigen processing in immune tolerance and inflammation
Immunogenetics (2019)
-
Mechanisms underlying the lack of endogenous processing and CLIP-mediated binding of the invariant chain by HLA-DP84Gly
Scientific Reports (2018)
-
Autoimmune T cell recognition of alternative-reading-frame-encoded peptides
Nature Medicine (2017)