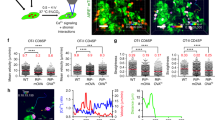
Abstract
In the steady state, dendritic cells (DCs) in the lymph node induce T cell tolerance to self antigens. Innate signals trigger the maturation of tissue DCs, which migrate into lymph nodes and activate T cells. To examine DCs in vivo, we produced transgenic mice whose DCs expressed enhanced yellow fluorescent protein. Two-photon microscopy of lymph nodes in live mice showed that most of the steady-state DCs were enmeshed in an extensive network and remained in place while actively probing adjacent T cells with their processes. Mature DCs were more motile than steady-state DCs and were rapidly dispersed and integrated into the sessile network, facilitating their interaction with migrating T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Banchereau, J., Pascual, V. & Palucka, K.A. Autoimunity through cytokine-induced dendritic cell activation. Immunity 20, 539–550 (2004).
Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20, 621–667 (2002).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
Liu, Y.J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106, 259–262 (2001).
Mellman, I. & Steinman, R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 (2001).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Itano, A.A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Manickasingham, S. & Reis e Sousa, C. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J. Immunol. 165, 5027–5034 (2000).
Inaba, K. et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188, 2163–2173 (1998).
Belz, G.T. et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA 101, 8670–8675 (2004).
Cyster, J.G. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J. Exp. Med. 189, 447–450 (1999).
Crowley, M., Inaba, K. & Steinman, R.M. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J. Exp. Med. 172, 383–386 (1990).
Bujdoso, R., Hopkins, J., Dutia, B.M., Young, P. & McConnell, I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J. Exp. Med. 170, 1285–1301 (1989).
Liu, L.M. & MacPherson, G.G. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J. Exp. Med. 177, 1299–1307 (1993).
Holt, P.G., Schon-Hegrad, M.A. & Oliver, J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J. Exp. Med. 167, 262–274 (1988).
Vermaelen, K.Y., Carro-Muino, I., Lambrecht, B.N. & Pauwels, R.A. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193, 51–60 (2001).
Huang, F.P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Belz, G.T. et al. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104 (2002).
Bousso, P. & Robey, E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 4, 579–585 (2003).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Miller, M.J., Hejazi, A.S., Wei, S.H., Cahalan, M.D. & Parker, I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. USA 101, 998–1003 (2004).
Miller, M.J., Safrina, O., Parker, I. & Cahalan, M.D. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 200, 847–856 (2004).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Brocker, T., Riedinger, M. & Karjalainen, K. Driving gene expression specifically in dendritic cells. Adv. Exp. Med. Biol. 417, 55–57 (1997).
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Wilson, N.S. et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102, 2187–2194 (2003).
Shortman, K. & Liu, Y.J. Mouse and human dendritic cell subtypes. Nature Rev. Immunol. 2, 151–161 (2002).
Miller, M.J., Wei, S.H., Cahalan, M.D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. USA 100, 2604–2609 (2003).
Kang, Y.S. et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15, 177–186 (2003).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Miller, M.J., Wei, S.H., Parker, I. & Cahalan, M.D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997).
Jacob, J., Kassir, R. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173, 1165–1175 (1991).
Liu, Y.J., Zhang, J., Lane, P.J., Chan, E.Y. & MacLennan, I.C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Euro. J. Immunol. 21, 2951–2962 (1991).
Friess, A. Interdigitating reticulum cells in the popliteal lymph node of the rat. An ultrastructural and cytochemical study. Cell Tissue Res. 170, 43–60 (1976).
Fossum, S. Lymph-borne dendritic leucocytes do not recirculate, but enter the lymph node paracortex to become interdigitating cells. Scand. J. Immunol. 27, 97–105 (1988).
Takeda, S., Rodewald, H.R., Arakawa, H., Bluethmann, H. & Shimizu, T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity 5, 217–228 (1996).
Kirberg, J., Berns, A. & von Boehmer, H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J. Exp. Med. 186, 1269–1275 (1997).
Inaba, K. et al. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J. Exp. Med. 186, 665–672 (1997).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Thery, C. et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147, 599–610 (1999).
Kaldjian, E.P., Gretz, J.E., Anderson, A.O., Shi, Y. & Shaw, S. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int. Immunol. 13, 1243–1253 (2001).
Katakai, T., Hara, T., Sugai, M., Gonda, H. & Shimizu, A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200, 783–795 (2004).
Benvenuti, F. et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 305, 1150–1153 (2004).
Hadjantonakis, A.K., Macmaster, S. & Nagy, A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2, 11 (2002).
Efron, B. Bootstrap methods: another look at the jackknife. Ann. Statist. 7, 1–26 (1979).
Acknowledgements
We thank R. Steinman for discussions and W. Gan for guidance on two-photon microscopy. Supported by the National Institutes of Health (AI55037 to M.L.D. and AI051573 to M.C.N.), Irene Diamond Foundation (M.L.D.), Rothschild Foundation (G.S.), Medical Scientist Training Program (GM07739 to R.L.L.), German Research Foundation (DU 548/1-1 to D.P.) and Howard Hughes Medical Institute (M.C.N.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Flow cytometric analysis of EYFP cells visible by two-photon microscopy. (PDF 366 kb)
Supplementary Fig. 2
Quantitative analysis of DC movement. (PDF 500 kb)
Supplementary Fig. 3
CD11c+ DCs form clusters with T and B cells in the steady state. (PDF 2360 kb)
Supplementary Video 1
Z-stack of two-photon images taken at depths of 0-300 μm through a B cell follicle in the LN of a living mouse. EYFP DCs are green, adoptively transferred EGFP B cells are false-colored cyan. Four populations of DCs are visible. Bright extended subcapsular DCs (arrows); dimmer DCs in a network surrounding the follicle; scattered compact DCs in the follicles (circles) and an extensive network of DCs in the T cell zone of the paracortex. (MOV 3861 kb)
Supplementary Video 2
Three-dimensional reconstruction of the different DC populations around a B cell follicle based on the data presented in Video 1. Colors are as in Video 1. (MOV 3453 kb)
Supplementary Video 3
No close association of resident CD11c-YFP cells with HEVs. Blood vessels were visualized with 66 kDa TRITC-dextran. The HEVs (crossing from top left to bottom right), can be distinguished from arterioles (crossing in the perpendicular direction) based on their irregular epithelium and the shadows of lymphocytes slowly rolling along it. YFP cellular debris is seen on the middle left. A mixture of slowly moving and sessile CD11c-YFP cells is seen scattered in the general area of the HEVs, but not immediately juxtaposed to them. Also seen are CFP lymphocytes (cyan) which exhibit a tighter morphology than DCs and can reach higher maximum speeds. (MOV 2956 kb)
Supplementary Video 4
Perifollicular DCs in relation to the LN capsule. A 3-dimensional reconstruction of a lymph node, showing the spatial relation of the perifollicular network of EYFP DCs (green) to the fibrous capsule (blue, second harmonics signal from collagen). (MOV 2480 kb)
Supplementary Video 5
Subcapsular sinus DCs and macrophages. Subcapsular sinus EYFP DCs (green) navigating among the less mobile macrophages, visualized by their phagocytosis of subcutaneously injected 66 kDa rhodamine-dextran (red). As in most videos below, a two-dimensional projection of a 50 μm thick volume is shown. (MOV 2793 kb)
Supplementary Video 6
Subcapsular DCs probing movement. At higher resolution, the probing movement of subcapsular DCs can be better appreciated. Note the characteristic ruffle shaped extensions of these cells. Dimmer, less motile perifollicular DCs are seen in the background. (MOV 3227 kb)
Supplementary Video 7
Tissue DC crawling. EYFP DCs (green) show fast crawling on the serosal surface lining the fat pads that neighbor the inguinal LN. Intravenously injected rhodamine dextran (red) flows in thin capillaries, bright red spots represent endocytosed dextran within endothelial cells. The morphology of these cells resembles that of subcapsular DCs in the LN. (MOV 1642 kb)
Supplementary Video 8
T cell zone DCs. EYFP DCs (green) form an extensive network in the T cell zone of the LN. Note that the DCs exhibit extensive probing movements but show little crawling. (MOV 3825 kb)
Supplementary Video 9
T cell zone DC network dynamics. A time sequence depicting a two-dimensional projection of a 50 μm volume in the interface of the T cell and B cell zones. The T cell zone is located below and to the left of the B cell follicle. The behavior of CD11c-EYFP DCs (green) in the network was followed. The great majority of the cells are laterally stable, exhibiting only probing movement, but occasionally a cell could be seen repositioning within the network (red circles). Adoptively transferred EGFP B cells are false-colored cyan. (MOV 2577 kb)
Supplementary Video 10
Different movement patterns of follicular and perifollicular DCs. Different behaviors of EYFP DCs (green) and ECFP B cells (cyan) in a B cell follicle (top right) and the perifollicular network (bottom left). Whereas some crawling movement is observed in the follicle, the DCs in the perifollicular network appear more dendritic and mainly probe with their processes. (MOV 1039 kb)
Supplementary Video 11
DC cluster Z stack. A Z-stack (45 μm deep, at 1 μm intervals) through several DC clusters in the T-B interface area. Each cluster is made up of numerous tightly apposed DCs enveloping lymphocytes. (MOV 2407 kb)
Supplementary Video 12
The behavior of DC clusters in the T-B interface zone (50 μm thick volume). Cluster position was stable for the duration and remained the same when the area was imaged again 4 hours later. DCs can be seen joining (blue circles) or leaving (red circles) the cluster. The shadows of lymphocytes can be seen drawn into the clusters enveloped in DC processes (yellow circles). (MOV 3422 kb)
Supplementary Video 13
Three-dimensional reconstruction of a DC cluster from the T-B interface area. The source data are confocal images that optically sectioned an immunofluorescently stained frozen section. EYFP is green, CD3 is red, and B220 is blue. Several DCs form a cluster that encloses numerous B and T cells. (MOV 2920 kb)
Supplementary Video 14
Resident LN DCs vs. transferred mature DCs of splenic origin. Transferred ECFP+ DCs (cyan), immunomagnetically purified from the spleen of a mouse treated with a FLT3-L-secreting tumor are seen here moving among resident CD11c-EYFP cells (yellow). The sequence was recorded at a depth of 180-230 μm 48 h after cell transfer. In this particular field, transferred cells crawled slightly faster than residents. Most of the movement consists of process probing, with cells occasionally flowing their soma and nucleus into one of the processes. To emphasize cell morphology, the CFP channel is shown alone on the right. (MOV 2619 kb)
Rights and permissions
About this article
Cite this article
Lindquist, R., Shakhar, G., Dudziak, D. et al. Visualizing dendritic cell networks in vivo. Nat Immunol 5, 1243–1250 (2004). https://doi.org/10.1038/ni1139
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1139
This article is cited by
-
Q fever immunology: the quest for a safe and effective vaccine
npj Vaccines (2023)
-
Monitoring Immune Cell Function Through Optical Imaging: a Review Highlighting Transgenic Mouse Models
Molecular Imaging and Biology (2022)
-
Behavioural immune landscapes of inflammation
Nature (2022)
-
Specialized transendothelial dendritic cells mediate thymic T-cell selection against blood-borne macromolecules
Nature Communications (2021)
-
FcγR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity
Nature Communications (2021)