Abstract
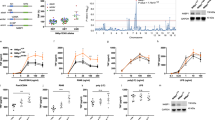
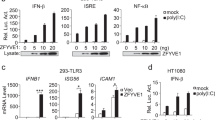
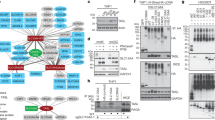
Stimulation of Toll-like receptors (TLRs) initiates potent innate immune responses through Toll–interleukin 1 receptor (TIR) domain–containing adaptors such as MyD88 and Trif. Analysis of Trif-deficient mice has shown that TLR3-dependent activation of the transcription factor NF-κB by the TLR3 ligand double-stranded RNA is Trif dependent. Here we investigated the 'downstream' signaling events that regulate TLR3-dependent Trif-induced NF-κB activation. Trif recruited the kinases receptor interacting protein (RIP)-1 and RIP3 through its RIP homotypic interaction motif. In the absence of RIP1, TLR3-mediated signals activating NF-κB, but not the kinase JNK or interferon-β, were abolished, suggesting that RIP1 mediates Trif-induced NF-κB activation. In contrast, the presence of RIP3 negatively regulated the Trif-RIP1–induced NF-κB pathway. Therefore, in contrast to other TLRs, which use interleukin 1 receptor-associated kinase (IRAK) proteins to activate NF-κB, TLR 3-induced NF-κB activation is dependent on RIP kinases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aderem, A. & Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000).
Rock, F.L., Hardiman, G., Timans, J.C., Kastelein, R.A. & Bazan, J.F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95, 588–593 (1998).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001).
O'Neill, L.A. & Dinarello, C.A. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol. Today 21, 206–209 (2000).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Hoshino, K. et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162, 3749–3752 (1999).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Kawai, T. et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167, 5887–5894 (2001).
Hoshino, K., Kaisho, T., Iwabe, T., Takeuchi, O. & Akira, S. Differential involvement of IFN-β in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14, 1225–1231 (2002).
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nat. Immunol. 4, 161–167 (2003).
Yamamoto, M. et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 (2002).
Yamamoto, M. et al. TRAM is specifically involved in the Toll-like receptor 4–mediated MyD88-independent signaling pathway. Nat. Immunol. 4, 1144–1150 (2003).
Oshiumi, H. et al. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-β. J. Biol. Chem. 278, 49751–49762 (2003).
Fitzgerald, K.A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Sato, S. et al. Toll/IL-1 Receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171, 4304–4310 (2003).
Hoebe, K. et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424, 743–748 (2003).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Lomaga, M.A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Hsu, H., Huang, J., Shu, H.B., Baichwal, V. & Goeddel, D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Sun, X. et al. RIP3, a novel apoptosis-inducing kinase. J. Biol. Chem. 274, 16871–16875 (1999).
Yu, P.W. et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NF-κB. Curr. Biol. 9, 539–542 (1999).
Sun, X., Yin, J., Starovasnik, M.A., Fairbrother, W.J. & Dixit, V.M. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 277, 9505–9511 (2002).
Kelliher, M.A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998).
Xu, Y. et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000).
Maniatis, T. et al. Structure and function of the interferon-β enhanceosome. Cold Spring Harb. Symp. Quant. Biol. 63, 609–620 (1998).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Meylan, E., Martinon, F., Thome, M., Gschwendt, M. & Tschopp, J. RIP4 (DIK/PKK), a novel member of the RIP kinase family, activates NF-κB and is processed during apoptosis. EMBO Rep. 3, 1201–1208 (2002).
Janssens, S. & Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11, 293–302 (2003).
Kobayashi, K. et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110, 191–202 (2002).
Cusson, N., Oikemus, S., Kilpatrick, E.D., Cunningham, L. & Kelliher, M. The death domain kinase RIP protects thymocytes from tumor necrosis factor receptor type 2-induced cell death. J. Exp. Med. 196, 15–26 (2002).
Thome, M. et al. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 8, 885–888 (1998).
Acknowledgements
We thank S. Hertig, N. Olivos, H. Everett, L. Agostini, M. Thome and B. Beutler for technical support, constructs and discussions. Supported by the Swiss National Science Foundation and Commission of Technology and Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Meylan, E., Burns, K., Hofmann, K. et al. RIP1 is an essential mediator of Toll-like receptor 3–induced NF-κB activation. Nat Immunol 5, 503–507 (2004). https://doi.org/10.1038/ni1061
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1061
This article is cited by
-
Caspase cleavage of RIPK3 after Asp333 is dispensable for mouse embryogenesis
Cell Death & Differentiation (2024)
-
5-Iodotubercidin sensitizes cells to RIPK1-dependent necroptosis by interfering with NFκB signaling
Cell Death Discovery (2023)
-
Rubiarbonol B induces RIPK1-dependent necroptosis via NOX1-derived ROS production
Cell Biology and Toxicology (2023)
-
Functions of the RIP kinase family members in the skin
Cellular and Molecular Life Sciences (2023)
-
Lipopolysaccharide induced altered signaling pathways in various neurological disorders
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)