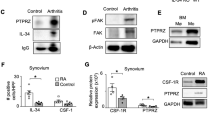
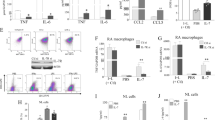
Abstract
The nonapoptotic functions of Fas ligation are incompletely characterized. In contrast to expectations, we show here that Fas-deficient mice developed less-severe collagen-induced arthritis than did control mice. Despite having milder arthritis, Fas-deficient mice had more of the critical pro-inflammatory mediator interleukin-1β (IL-1β) in their joints, suggesting inefficient activation through IL-1 receptor 1 (IL-1R1) when Fas signaling is deficient. In primary human macrophages and macrophages from Fas- or Fas ligand (FasL)-deficient mice, interruption of Fas-FasL signaling suppressed nuclear factor-κB activation and cytokine expression induced by IL-1β and lipopolysaccharide. This cross-talk was mediated by the Fas-associated death domain through interaction with myeloid differentiation factor 88. These observations document a unique mechanism whereby Fas-FasL interactions enhance activation through the IL-1R1 or Toll-like receptor 4 pathway, which may contribute to the pathogenesis of chronic arthritis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mulherin, D., Fitzgerald, O. & Bresnihan, B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 39, 115–124 (1996).
Tak, P.P. et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 40, 217–225 (1997).
Feldmann, M., Brennan, F.M. & Maini, R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14, 397–440 (1996).
Bathon, J.M. et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N. Engl. J. Med. 343, 1586–1593 (2000).
Lipsky, P.E. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 343, 1594–1602 (2000).
Cohen, S. et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 614–624 (2002).
Wendling, D., Racadot, E. & Wijdenes, J. Treatment of severe rheumatoid arthritis by anti-interleukin 6 monoclonal antibody. J. Rheumatol. 20, 259–262 (1993).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Termeer, C. et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195, 99–111 (2002).
Okamura, Y. et al. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276, 10229–10233 (2001).
Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164, 558–561 (2000).
Palmer, G. et al. Mice transgenic for intracellular interleukin-1 receptor antagonist type 1 are protected from collagen-induced arthritis. Eur. J. Immunol. 33, 434–440 (2003).
Williams, R.O., Marinova-Mutafchieva, L., Feldmann, M. & Maini, R.N. Evaluation of TNF-α and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-α/anti-CD4 therapy. J. Immunol. 165, 7240–7245 (2000).
Yoshino, S., Sasatomi, E., Mori, Y. & Sagai, M. Oral administration of lipopolysaccharide exacerbates collagen-induced arthritis in mice. J. Immunol. 163, 3417–3422 (1999).
Choe, J.Y., Crain, B., Wu, S.R. & Corr, M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by Toll-like receptor 4 signaling. J. Exp. Med. 197, 537–542 (2003).
Chinnaiyan, A.M., O'Rourke, K., Tewari, M. & Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505–512 (1995).
Cohen, P.L. & Eisenberg, R.A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9, 243–269 (1991).
Kataoka, T. et al. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr. Biol. 10, 640–648 (2000).
Hu, W.H., Johnson, H. & Shu, H.B. Activation of NF-κB by FADD, Casper, and caspase-8. J. Biol. Chem. 275, 10838–10844 (2000).
Ahn, J.H. et al. Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-κB-dependent gene expression. J. Biol. Chem. 276, 47100–47106 (2001).
Kabra, N.H., Kang, C., Hsing, L.C., Zhang, J. & Winoto, A. T cell-specific FADD-deficient mice: FADD is required for early T cell development. Proc. Natl. Acad. Sci. USA 98, 6307–6312 (2001).
Zhang, J., Cado, D., Chen, A., Kabra, N.H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 (1998).
Perlman, H. et al. Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1β-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis. Arthritis Rheum. 44, 21–30 (2001).
Perlman, H. et al. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J. Exp. Med. 190, 1679–1688 (1999).
Yao, P.M. & Tabas, I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J. Biol. Chem. 275, 23807–23813 (2000).
Georganas, C. et al. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-κB but not C/EBPβ or c-Jun. J. Immunol. 165, 7199–7206 (2000).
Chinnaiyan, A.M. et al. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J. Biol. Chem. 271, 4961–4965 (1996).
Eickelberg, O. et al. Calcium channel blockers activate the interleukin-6 gene via the transcription factors NF-IL6 and NF-κB in primary human vascular smooth muscle cells. Circulation 99, 2276–2282 (1999).
Bradham, C.A. et al. The mitochondrial permeability transition is required for tumor necrosis factor α-mediated apoptosis and cytochrome c release. Mol. Cell. Biol. 18, 6353–6364 (1998).
Aliprantis, A.O., Yang, R.B., Weiss, D.S., Godowski, P. & Zychlinsky, A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 19, 3325–3336 (2000).
Scaffidi, C., Krammer, P.H. & Peter, M.E. Isolation and analysis of components of CD95 (APO-1/Fas) death-inducing signaling complex. Methods: A Companion to Methods Enzymol. 17, 287–291 (1999).
Pope, R.M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat. Rev. Immunol. 2, 527–535 (2002).
Alonzi, T. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J. Exp. Med. 187, 461–468 (1998).
Varfolomeev, E.E. et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Yeh, W.C. et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12, 633–642 (2000).
Chaudhary, P.M. et al. Activation of the NF-κB pathway by caspase 8 and its homologs. Oncogene 19, 4451–4460 (2000).
Ponton, A., Clement, M.V. & Stamenkovic, I. The CD95 (APO-1/Fas) receptor activates NF-κB independently of its cytotoxic function. J. Biol. Chem. 271, 8991–8995 (1996).
Russo, M.P., Bennett, B.L., Manning, A.M., Brenner, D.A. & Jobin, C. Differential requirement for NF-κB-inducing kinase in the induction of NF-κB by IL-1β, TNF-α, and Fas. Am. J. Physiol. Cell Physiol. 283, C347–357 (2002).
Pagliari, L.J., Perlman, H., Liu, H. & Pope, R.M. Macrophages require constitutive NF-κB activation to maintain A1 expression and mitochondrial homeostasis. Mol. Cell. Biol. 20, 8855–8865 (2000).
Park, D.R. et al. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J. Immunol. 170, 6209–6216 (2003).
Shain, K.H., Landowski, T.H. & Dalton, W.S. Adhesion-mediated intracellular redistribution of c-Fas-associated death domain-like IL-1-converting enzyme-like inhibitory protein-long confers resistance to CD95-induced apoptosis in hematopoietic cancer cell lines. J. Immunol. 168, 2544–2553 (2002).
Aoudjit, F. & Vuori, K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 152, 633–643 (2001).
Liu, H. et al. TNF-α gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPβ. J. Immunol. 164, 4277–4285 (2000).
Bannerman, D.D., Tupper, J.C., Kelly, J.D., Winn, R.K. & Harlan, J.M. The Fas-associated death domain protein suppresses activation of NF-κB by LPS and IL-1β. J. Clin. Invest. 109, 419–425 (2002).
Wajant, H. et al. Dominant-negative FADD inhibits TNFR60-, Fas/Apo1- and TRAIL-R/Apo2-mediated cell death but not gene induction. Curr. Biol. 8, 113–116 (1998).
Hsu, H., Shu, H.B., Pan, M.G. & Goeddel, D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84, 299–308 (1996).
Fukui, M., Imamura, R., Umemura, M., Kawabe, T. & Suda, T. Pathogen-associated molecular patterns sensitize macrophages to Fas ligand-induced apoptosis and IL-1β release. J. Immunol. 171, 1868–1874 (2003).
Rescigno, M. et al. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1β, and the production of interferon γ in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. J. Exp. Med. 192, 1661–1668 (2000).
Singer, G.G., Carrera, A.C., Marshak-Rothstein, A., Martinez, C. & Abbas, A.K. Apoptosis, Fas and systemic autoimmunity: the MRL-lpr/lpr model. Curr. Opin. Immunol. 6, 913–920 (1994).
Wooley, P.H., Luthra, H.S., Stuart, J.M. & David, C.S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J. Exp. Med. 154, 688–700 (1981).
Ibrahim, S.M., Koczan, D. & Thiesen, H.J. Gene-expression profile of collagen-induced arthritis. J. Autoimmun. 18, 159–167 (2002).
Liu, H., Perlman, H., Pagliari, L.J. & Pope, R.M. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J. Exp. Med. 194, 113–126 (2001).
Scaffidi, C. et al. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274, 22532–22538 (1999).
Acknowledgements
We thank M.E. Peter and V.M. Dixit for providing the FADD and dominant negative FADD plasmids; J. Karras for providing the antisense FADD deoxyoligonucleotides; and R. Medzhitov for providing the MyD88 plasmid. We also thank H. Perlman, P.H. Stern, N.A. Clipstone and A. Lin for critical review of the manuscript; M.E. Peter for advice on the immunoprecipitation experiments; C.J. Zander for technical assistance; and H.-J. Kreutzer for help evaluating joint histopathology. Supported by the National Institutes of Health (R01-AR049217) and The Veterans Administration Research Service (Merit Review), the National and Greater Chicagoland Chapters of the Arthritis Foundation and Deutsche Forschungsgemeinschaft (IB 24/3-1 to S.M.I.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ma, Y., Liu, H., Tu-Rapp, H. et al. Fas ligation on macrophages enhances IL-1R1–Toll-like receptor 4 signaling and promotes chronic inflammation. Nat Immunol 5, 380–387 (2004). https://doi.org/10.1038/ni1054
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1054
This article is cited by
-
Phosphopeptides P140 cause oxidative burst responses of pulmonary macrophages in an imiquimod-induced lupus model
Molecular Biomedicine (2023)
-
A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma
Journal of Neuroinflammation (2019)
-
The caspase-8/RIPK3 signaling axis in antigen presenting cells controls the inflammatory arthritic response
Arthritis Research & Therapy (2017)
-
HMGB1 release triggered by the interaction of live retinal cells and uveitogenic T cells is Fas/FasL activation-dependent
Journal of Neuroinflammation (2015)
-
DED or alive: assembly and regulation of the death effector domain complexes
Cell Death & Disease (2015)