Abstract
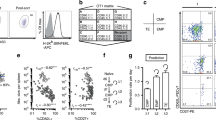
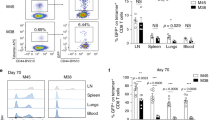
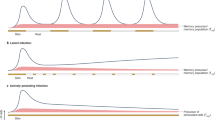
We studied the influence of memory T cell properties on the efficiency of secondary immune responses by comparing the in vivo immune response of the same numbers of T cell receptor (TCR) transgenic (Tg) naïve and memory T cells. Compared to naïve Tg cells, memory cells divided after a shorter lag time; had an increased division rate; a lower loss rate; and showed more rapid and efficient differentiation to effector functions. We found that initial naïve T cell priming resulted in cells expressing mutually exclusive effector functions, whereas memory T cells were multifunctional after reactivation, with each individual cell expressing two to three different effector functions simultaneously. These special properties of memory T cells ensure the immediate control of reinfection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Owen, J.A., Allouche, M. & Doherty, P.C. Limiting dilution analysis of the specificity of influenza-immune cytotoxic T cells. Cell. Immunol. 67, 49–59 (1982).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Doherty, P.C., Topham, D.J. & Tripp, R.A. Establishment and persistence of virus-specific CD4+ and CD8+ T cells memory. Immunol. Rev. 150, 23–44 (1996).
Flynn, K. J. et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8, 683–691 (1998).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Busch, D.H. & Pamer, E.G. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189, 701–10 (1999).
McHeyzer-Williams, L.J., Panus, J.F., Mikszta, J.A. & McHeyzer-Williams, M.G. Evolution of antigen-specific T cell receptors in vivo: preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J. Exp. Med. 189, 1823–38 (1999).
Savage, P.A., Boniface, J.J. & Davis, M.M. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10, 485–921 (1999).
Selin, L. K. et al. Attrition of T cell memory: Selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11, 733–742 (1999).
Budd, R.C. et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 138, 3120–3129 (1987).
Bruno, L., Kirberg, J. & von Boehmer, H. On the cellular basis of immunological T cell memory. Immunity 2, 37–43 (1995).
Tanchot, C. et al. Differential requirements for survival and proliferation of CD8 naïve or memory T cells. Science 276, 2057–2062 (1997).
Curtsinger, J.M., Lins, D.C. & Mescher, M.F. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells (CD44low, Ly-6C-) to TcR/CD8 signaling in response to antigen. J. Immunol. 160, 3236–3243 (1998).
Tanchot, C. et al. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity 8, 581–590 (1998).
Cho, B. K. et al. Functional differences between memory and naive CD8 T cells. Proc. Natl Acad. Sci. USA 96, 2976–2981 (1999).
Garcia, S., DiSanto, J. & Stockinger, B. Following the Development of a CD4 T cell response in vivo: from activation to memory formation. Immunity 11, 163–171 (1999).
Zimmermann, C., Prevost-Blondel, A., Blaser, C. & Pircher, H. Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences. Eur. J. Immunol. 29, 284–290 (1999).
Bachmann, M.F., Barner, M., Viola, A. & Kopf, M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29, 291–299 (1999).
Tanchot, C. & Rocha, B. The organization of mature T cell pools. Immunol. Today 19, 575–579 (1998).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Kisielow, P et al. Tolerance in T cell receptor transgenic mice involves deletion of nonmature CD4+CD8+ thymocytes. Nature 333, 742–746 (1988).
Rocha, B., Grandien, A. & Freitas, A.A. Anergy and exhaustion are independent mechanisms of peripheral tolerance. J. Exp. Med. 181, 993–1003 (1995).
McLean, A. R. et al. Resourse competition as a mechanism for B cell homeostasis. Proc. Natl Acad. Sci. USA 94, 5792–5797 (1997).
Borghans, J.A.M., Taams, L.S., Wauben, M.H.M. & De Boer, R. Competition for antigenic sites during T cell proliferation: a mathematical interpretation of in vitro data. Proc. Natl Acad. Sci. USA 96, 10782–10787 (1999).
Tripp, R.A., Lahti, J.M. & Doherty, P.C. Laser light suicide of proliferating virus-specific CD8+ T cells in an in vivo response. J. Immunol. 155, 3719–3721 (1995).
Sprent, J., Tough, D.F. & Sun, S. Factors controlling the turnover of T memory cells. Immunol. Rev. 156, 79–85 (1997).
Leslie, P.H. Some further notes on the use of matrices in population mathematics. Biometrika 35, 213–245 (1948).
De Boer, R.J. & Noest, A.J. T cell renewal rates, telomerase and telomere length shortening. J. Immunol. 160, 5832–5837 (1998).
Karulin, A.Y., Hesse, M.D., Tary-Lehmann, M. & Lehmann, P.V. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J. Immunol. 164, 1862–1872 (2000).
Valitutti, S. et al. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 11, 148–151 (1995).
Bachmann, M.F. et al. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J. Exp. Med. 189, 1521–1529 (1999).
Mackay, C.R. Migration pathways and immunologic memory among T lymphocytes. Semin. Immunol. 4, 51–58 (1992).
Brown, K.E. et al. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol. Cell. 3, 207–217 (1999).
Agarwal, S. & Rao, A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity 9, 765–775 (1998).
Lewin, B. The mystique of epigenetics. Cell 93, 301–303 (1998).
Fitzpatrick, D.R., Shirley, K.M. & Kelso, A. Stable epigenetic inheritance of regional IFN-gamma promoter demethylation in CD44 high CD8+ T lymphocytes. J. Immunol. 162, 5053–5057 (1999).
Swain, S.L. et al. From naive to memory T cells. Immunol Rev. 150, 143–167 (1996).
Gett, A. & Hodgkin, P.D. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc. Natl Acad. Sci. USA 95, 9488–9493 (1998).
Bird, J.J. et al. Helper T cell differentiation is controlled by the cell cycle. Immunity 9, 229–237 (1998).
Richter, A., Lohning, M. & Radbruch, A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell progression. J. Exp. Med. 190, 1439–1450 (1999).
Gudmundsdottir, H., Wells, A.D. & Turka, L.A. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J. Immunol. 162, 5212–5223 (1999).
Sad, S. & Mosmann, T.R. Single IL-2 secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J. Immunol. 153, 3514–3522 (1994).
Viola, A. & Lanzavecchia, A. T cell activation determined by T cell receptor number and tunable thresholds. Science 273, 104–106 (1996).
Itoh, Y. & Germain, R.N. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogenety for individual cytokine responses of CD4+ T cells. J. Exp. Med. 186, 757–766 (1997).
Waldorp, S.L., Davis, K.A., Maino, V.C. & Picker, L.J. Normal human CD4+ memory cells display broad heterogenety in their activation threshold for cytokine synthesis. J. Immunol. 161, 5282–5295 (1998).
Weaver, C.T. Heterogeneity in the clonal T cell response: implications for models of T cell activation and cytokine phenotype development. Immunol. Res. 17, 279–302 (1998).
Nutt, S.L., Heavey, B., Rolink, A.G. & Busslinger, M. Commitment to B-lymphoid lineage depends on the transcription factor Pax5. Nature 401, 556–562 (1999).
Selin, L.K. & Welsh, R.M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J. Immunol. 158, 5366–5373 (1997).
Opferman, J.T., Ober, B.T. & Ashton-Rickardt, P.G. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745–1748 (1999).
Naramura, M., Hu, R. & Gu, H. Mice with a fluorescent marker for interleukin 2 gene activation. Immunity 9, 209–216 (1998).
Saparov, A. et al. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity 11, 271–280 (1999).
Malissen, M. et al. Altered T cell development in mice with a targeted mutation of the CD3 ɛ gene. EMBO J. 14, 4641–4653 (1995).
Teh, H.S. et al. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature 335, 229–233 (1988).
Lyons, A.B. & Parish, C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 171, 131–137 (1994).
Tafuri, A. et al. Combination of hematopoietic growth factors containing IL-3 induce acute myeloid leukemia cell sensitization to cycle specific and cycle non-specific drugs. Leukemia 8, 749–757 (1994).
Loffert, D., Ehlich, A., Muller, W. & Rajewsky, K. Surrogate light chain expression is required to establish immunoglobulin heavy chain allelic exclusion during early B cell development. Immunity 4, 133–144 (1996).
Pannetier, C. et al. Quantitative titration of nucleic acids by enymatic amplification reactions run to saturation. Nucleic Acids Res. 21, 577–583 (1993).
Taswell, C. Limiting dilution assays for the determination of immunocompetent cell frequencies. III. Validity tests for the single-hit Poisson model. J. Immunol. Methods 72, 29–40 (1984).
Siminovitch, L., McCulloch, E.A. & Till, J E. The distribution of colony forming cells among spleen colonies. J. Cell Comp. Physiol. 62, 327–336 (1963).
Acknowledgements
We thank C. Garcia for cell sorting; A.M. Joret and S. Leaument for technical assistance; A. Le Campion for statistics; J. Di Santo, A.A. Freitas, D. Guy-Grand, A. Sarukhan and H. von Boehmer for reviewing the manuscript. This work was supported by grants from the National Association of AIDS Research, France. H.V.-F. was supported by a grant from Technology and Science Foundation, Praxis XXI, Portugal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veiga-Fernandes, H., Walter, U., Bourgeois, C. et al. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol 1, 47–53 (2000). https://doi.org/10.1038/76907
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76907
This article is cited by
-
A novel “prime and pull” strategy mediated by the combination of two dendritic cell-targeting designs induced protective lung tissue-resident memory T cells against H1N1 influenza virus challenge
Journal of Nanobiotechnology (2023)
-
The fellowship of regulatory and tissue-resident memory cells
Mucosal Immunology (2022)
-
Activated-memory T cells influence naïve T cell fate: a noncytotoxic function of human CD8 T cells
Communications Biology (2022)
-
Genome organization in immune cells: unique challenges
Nature Reviews Immunology (2019)
-
Regulation of T cell differentiation and function by epigenetic modification enzymes
Seminars in Immunopathology (2019)