Abstract
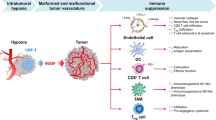
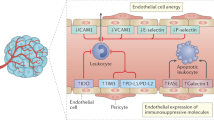
The tumor microenvironment confers profound resistance to anti-cancer immunotherapy. By targeting LIGHT, a member of the TNF superfamily of cytokines, to tumor vessels via a vascular targeting peptide (VTP), we developed a reagent with the dual ability to modulate the angiogenic vasculature and to induce tertiary lymphoid structures (TLSs). LIGHT-VTP triggered the influx of endogenous T cells into autochthonous or syngeneic tumors, which are resistant to immunotherapy. LIGHT-VTP in combination with checkpoint inhibition generated a large number of intratumoral effector and memory T cells with ensuing survival benefits, while the addition of anti-tumor vaccination achieved maximal therapeutic efficacy. Thus, the combination treatments stimulated the trafficking of pre-existing endogenous effector T cells as well as their intratumoral activation and were more successful than current immunotherapies, which fail due to tumor-intrinsic resistance mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Galon, J. et al. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 10, 1 (2012).
Gajewski, T.F. et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013).
Turley, S.J., Cremasco, V. & Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15, 669–682 (2015).
Johansson, A., Hamzah, J. & Ganss, R. More than a scaffold: Stromal modulation of tumor immunity. Biochim. Biophys. Acta 1865, 3–13 (2016).
Jain, R.K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014).
Dirkx, A.E. et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 63, 2322–2329 (2003).
Hamzah, J. et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453, 410–414 (2008).
Hamzah, J. et al. Vascular targeting of anti-CD40 antibodies and IL-2 into autochthonous tumors enhances immunotherapy in mice. J. Clin. Invest. 118, 1691–1699 (2008).
Johansson, A., Hamzah, J., Payne, C.J. & Ganss, R. Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc. Natl. Acad. Sci. USA 109, 7841–7846 (2012).
Shrimali, R.K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Dirkx, A.E. et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 20, 621–630 (2006).
Johansson-Percival, A. et al. Intratumoral LIGHT restores pericyte contractile properties and vessel integrity. Cell Rep. 13, 2687–2698 (2015).
Lu, T.T. & Browning, J.L. Role of the lymphotoxin/LIGHT system in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front. Immunol. 5, 47 (2014).
Browning, J.L. et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Dieu-Nosjean, M.C. et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 271, 260–275 (2016).
Kirk, C.J., Hartigan-O'Connor, D. & Mulé, J.J. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 61, 8794–8802 (2001).
Thompson, E.D., Enriquez, H.L., Fu, Y.X. & Engelhard, V.H. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J. Exp. Med. 207, 1791–1804 (2010).
Shields, J.D., Kourtis, I.C., Tomei, A.A., Roberts, J.M. & Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Joshi, N.S. et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak96 (2017).
Schmittnaegel, M. et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9, eaak9670 (2017).
Yu, P. et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 5, 141–149 (2004).
Chang, Y.H., Hsieh, S.L., Chao, Y., Chou, Y.C. & Lin, W.W. Proinflammatory effects of LIGHT through HVEM and LTbetaR interactions in cultured human umbilical vein endothelial cells. J. Biomed. Sci. 12, 363–375 (2005).
Ruoslahti, E., Bhatia, S.N. & Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 188, 759–768 (2010).
Johansson, A., Hamzah, J. & Ganss, R. License for destruction: tumor-specific cytokine targeting. Trends Mol. Med. 20, 16–24 (2014).
Mazzone, M. et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851 (2009).
Cantelmo, A.R. et al. Inhibition of the glycolytic activator PFKFB3 in Endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30, 968–985 (2016).
Messina, J.L. et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci. Rep. 2, 765 (2012).
Onder, L. et al. Endothelial cell-specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. J. Exp. Med. 210, 465–473 (2013).
Fan, L., Reilly, C.R., Luo, Y., Dorf, M.E. & Lo, D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J. Immunol. 164, 3955–3959 (2000).
Förster, I., Hirose, R., Arbeit, J.M., Clausen, B.E. & Hanahan, D. Limited capacity for tolerization of CD4+ T cells specific for a pancreatic beta cell neo-antigen. Immunity 2, 573–585 (1995).
Garbi, N., Arnold, B., Gordon, S., Hämmerling, G.J. & Ganss, R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J. Immunol. 172, 5861–5869 (2004).
Curran, M.A., Montalvo, W., Yagita, H. & Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 107, 4275–4280 (2010).
Page, D.B., Postow, M.A., Callahan, M.K., Allison, J.P. & Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 65, 185–202 (2014).
Ganss, R., Ryschich, E., Klar, E., Arnold, B. & Hämmerling, G.J. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 62, 1462–1470 (2002).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013).
Topalian, S.L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030 (2014).
Hiraoka, N. et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer 112, 1782–1790 (2015).
Bento, D.C. et al. High endothelial venules are rare in colorectal cancers but accumulate in extra-tumoral areas with disease progression. OncoImmunology 4, e974374 (2015).
Fisher, D.T. et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J. Clin. Invest. 121, 3846–3859 (2011).
Peske, J.D. et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat. Commun. 6, 7114 (2015).
Guedj, K. et al. M1 macrophages act as LTβR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc. Res. 101, 434–443 (2014).
Moynihan, K.D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
Ganss, R. & Hanahan, D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 58, 4673–4681 (1998).
Schrama, D. et al. Targeting of lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 14, 111–121 (2001).
Maldonado, L. et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci. Transl. Med. 6, 221ra13 (2014).
Lutz, E.R. et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res. 2, 616–631 (2014).
Joyce, J.A. et al. Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell 4, 393–403 (2003).
Agemy, L. et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 108, 17450–17455 (2011).
Acknowledgements
We thank S. Tanz (The University of Western Australia) for assistance with laser-dissection microscopy; J. Hamzah (Harry Perkins Institute of Medical Research) for the LIGHT expression plasmid; E. Ingley (Harry Perkins Institute of Medical Research) for the pET-44a plasmid containing a tobacco etch virus cleavage site; and D. Hanahan (Swiss Institute for Experimental Cancer Research) for rabbit polyclonal antibody to Tag. Supported by the National Health and Medical Research Council (APP1042446 and APP1122108 to R.G.), the Cancer Council of Western Australia (APP1098579 to R.G.; fellowship to A.J.-P.) and Woodside Energy (fellowship to R.G.).
Author information
Authors and Affiliations
Contributions
A.J.-P., B.H., Z.-J.L., A.K. and I.L. performed experiments and analyzed data; K.R. and J.L. performed experiments; A.J.-P., I.L. and R.G. designed experiments and interpreted data; and A.J.-P. and R.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Recombinant LIGHT-VTP induces pericyte contractility and endothelial cell activation.
(a) Western blot showing purification steps for full length murine LIGHT-VTP (CGKRK) protein: (1) protein marker; (2) fusion protein expression in bacterial lysate; (3) column flow through; (4) column washing; (5) eluate from Ni-NTA column; (6) protein after dialysis; (7) released protein after tobacco edge virus (TEV) protease cleavage; (8) purified LIGHT-CGKRK at approximately 20 kDa (boxed); arrow points at bovine serum albumin (BSA) which was added for stability. (b) Histological quantification of CD31+ vessel diameters in untreated mice (n=4), or mice treated bi-weekly for 2 weeks with VTP (n=3) or 20 ng LIGHT-VTP (LV, n=3). **P=0.009, *P=0.003 (Student’s t-test). Scale bar, 50 μm. (c) Histological analysis of ICAM expression in untreated (n=4) and LV treatment groups (n=6). *P=0.003 (Student’s t-test). Scale bar, 50 μm. (d) Immunohistochemical quantification of pericyte (αSMA+) expression of contractile markers (calp, calponin; cald, caldesmon, arrows) and tumor-associated collagen I (col I) in treatment groups. Caldesmon, n=7 untr mice, n=5 LV treated mice; calponin, n=5 untr mice, n=7 LV treated mice; collagen I, n=9 untr mice, n=6 LV treated mice. ***P=0.0001, **P=0.008, *P=0.04, Student’s t-test. Scale bars, 50 μm. Data are presented as means ± s.d. and representative of three independent experiments.
Supplementary Figure 2 CCL21+ TLSs are induced by LIGHT-VTP or transfer of LIGHT-stimulated macrophages.
(a) Histological analysis of LV-induced TLS structures in RIP1-Tag5 tumors before and after laser microdissection and pressure catapulting (LMPC). Scale bar, 100 μm (upper), 150 μm (lower). (b) Immunohistochemical analysis of CCL21 expression in untreated or LV treated tumors in conjunction with MECA79+ HEV (middle) or CD68+ macrophage (right) stainings. Dashed lines show TLS. Depicted are CCL21-positive endothelial cells (arrows), HEV structure (filled arrow head) and macrophages associated with a blood vessel (open arrow head). Scale bar, 50 μm. (c) Relative mRNA in macrophage-depleted, naïve (left) or ConA-activated (right) splenocytes, n=3 C3H donor mice. (d) Schematic presentation of adoptive macrophage transfers (AdT) into tumor-bearing RIP1-Tag5 mice. (e) Quantification of B cells within 100 μm of MECA79+ vessels in tumors after macrophage transfer, *P=0.02, n=3 (Student’s t-test). (f) Histological analysis of CCL21 expression in tumors after transfer of PBS- or LV-stimulated macrophages (PBS MФ, LV MФ), or LV MФ transfer into T cell-depleted mice (αCD4/8). Dashed lines show TLS. Depicted are CCL21-positive endothelial cells (arrows) and macrophages (open arrow heads). Scale bar, 50 μm. Data are presented as mean ± s.d. and are representative of one experiment (c, e, f), two independent (b) and three independent experiments (a).
Supplementary Figure 3 LIGHT-VTP treatment induces infiltration of immune cells into RIP1-Tag5 tumors.
(a) Representative FACS plots showing gating strategy for Fig. 4a including Fluorescence Minus One (FMO) controls. (b) Macroscopic tumor appearance shown for individual RIP1-Tag5 mice at 30 weeks of age, representative images for untreated and LV treatment groups are shown.
Supplementary Figure 4 PDL-1 expression in RIP1-Tag5 tumors and therapeutic effects of checkpoint-blockade treatments.
(a) Histological analysis of PD-L1 expression in untreated RIP1-Tag5 tumors (n=4) and after a 2 week treatment with bi-weekly injections of 20 ng LIGHT-VTP (LV, n=3). Scale bar, 50 μm. Data are presented as mean ± s.d. (b) Survival analysis of RIP1-Tag5 mice treated with LV and control rat IgG2a antibody (n=8), αCTLA-4 (n=6) or αPD-1 (n=5) as single treatment modalities. (c) Macroscopic RIP1-Tag5 tumor appearance after 7 weeks of treatment with LV and control antibodies (LV+control abs) or LV combined with checkpoint inhibitors (LV+αCTLA-4/αPD-1). Representative images from individual mice are shown. Data represent two independent experiments.
Supplementary Figure 5 LIGHT-VTP and checkpoint blockade induce effector T cells in tumors and draining lymph nodes.
(a) Schematic outline of long term treatment of RIP1-Tag5 mice from 23 to 30 weeks with combination therapies. (b) FMO controls for the markers Ki67 and GrzB, representative contour plots. Corresponding data are shown in Fig. 6b. (c) FMO controls for the markers FoxP3 and CD25. Corresponding data are shown in Fig. 6c. (d) FACS quantification of % CD25+ FoxP3+ Treg cells of CD3+ CD4+ T cells in pancreatic tumor-draining lymph nodes of RIP1-Tag5 mice which were left untreated, or treated with LIGHT-VTP and control antibodies (LV+ctrl) or LV+αCTLA-4/αPD-1 antibodies (LV+αCTLA-4/αPD-1), n=3-6 mice (pooled into one group because of limited size of pancreas draining lymph nodes). (e) Ratio of intratumoral Ki67+ GrzB+ CD4+ or CD8+ effector T cells to CD25+ FoxP3+ Treg cells of CD3+ CD4+ T cells, n=4 for all CD4+ T cell analyses, n=5 for CD8+ T cells in LV+ctrl tumors, n=4 for CD8+ T cells in LV αCTLA-4/αPD-1 tumors, *P=0.021, **P=0.001 (Student’s t-test). (f) Quantification of Ki67+ GrzB+ effector CD4+ and CD8+ T cells of CD3+ T cells in tumor-draining lymph nodes from various treatment groups, and representative plots, n=3-6 mice (pooled into one group). Data represent two independent experiments.
Supplementary Figure 6 LIGHT-VTP and checkpoint blockade induce a switch from naive T cells to memory T cells.
(a) FACS quantification of % CD4+ or CD8+ naïve (CD44low CCR7+, upper graph) or memory (CD44+ CCR7-, lower graph) T cells of CD3+ T cells from untreated tumors (n=7), or tumors of LIGHT-VTP+control antibodies (LV+ctrl, n=5) or LV+αCTLA-4/αPD-1 treated mice (n=4 mice), and representative plots. *P=0.05, **P=0.01, Student’s t-test. (b) Quantification of % intratumoral CD4+ or CD8+ proliferating (Ki67+) T cells of memory (CD44+ CCR7-) CD3+ T cells from different treatment groups and representative plots. *P=0.02 (Student’s t-test). (c) FMO controls for CD44 and CCR7, representative FACS plots. (d) FMO controls for CD44, CCR7 and Ki67 stainings, representative FACS plots. Data are presented as mean ± s.d. and represent two independent experiments.
Supplementary Figure 7 LIGHT-VTP combination immunotherapies enhance survival and reduce tumor angiogenesis.
(a) Treatment scheme for bi-weekly LIGHT-VTP (LV) injections combined with adoptive T cell transfers (AdT) or anti-Tag/CpG-ODN vaccine with or without checkpoint blockade. (b) Survival analyses comparing LV+Tag-activated TCR T cell transfer (Tag T) (n=6, mean survival 34±2 weeks) with LV+ConA-activated C3H T cell transfer (ConA T) (n=5, 30±2 weeks). The LV group is shown as reference (original data in Fig. 4d). **P=0.008, *P=0.05 (log-rank test). Data are representative for one experiment. (c) Macroscopic tumor appearance at 29 weeks of age in RIP1-Tag5 groups treated with LV+vaccine or LV triple therapy + vaccine. Representative images from individual mice are shown.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–4 (PDF 1177 kb)
Rights and permissions
About this article
Cite this article
Johansson-Percival, A., He, B., Li, ZJ. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol 18, 1207–1217 (2017). https://doi.org/10.1038/ni.3836
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3836
This article is cited by
-
Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application
Molecular Cancer (2024)
-
Synergistic induction of tertiary lymphoid structures by chemoimmunotherapy in bladder cancer
British Journal of Cancer (2024)
-
Reprogramming systemic and local immune function to empower immunotherapy against glioblastoma
Nature Communications (2023)
-
Methylation across the central dogma in health and diseases: new therapeutic strategies
Signal Transduction and Targeted Therapy (2023)
-
The roles of tertiary lymphoid structures in chronic diseases
Nature Reviews Nephrology (2023)