Abstract
The transcription factor RORγt regulates differentiation of the TH17 subset of helper T cells, thymic T cell development and lymph-node genesis. Although elimination of RORγt prevents TH17 cell–mediated experimental autoimmune encephalomyelitis (EAE), it also disrupts thymocyte development, which could lead to lethal thymic lymphoma. Here we identified a two-amino-acid substitution in RORγt (RORγtM) that 'preferentially' disrupted TH17 differentiation but not thymocyte development. Mice expressing RORγtM were resistant to EAE associated with defective TH17 differentiation but maintained normal thymocyte development and normal lymph-node genesis, except for Peyer's patches. RORγtM showed less ubiquitination at Lys69 that was selectively required for TH17 differentiation but not T cell development. This study will inform the development of treatments that selectively target TH17 cell–mediated autoimmunity but do not affect thymocyte development or induce lymphoma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 2006).
Gaffen, S.L., Jain, R., Garg, A.V. & Cua, D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Codarri, L. et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
El-Behi, M. et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Ueda, E. et al. High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORgamma. Cancer Res. 62, 901–909 (2002).
Kurebayashi, S. et al. Retinoid-related orphan receptor gamma (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. USA 97, 10132–10137 (2000).
Chen, Y. et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326 (2006).
Eberl, G. et al. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Huh, J.R. & Littman, D.R. Small molecule inhibitors of RORγt: targeting Th17 cells and other applications. Eur. J. Immunol. 42, 2232–2237 (2012).
Burchill, M.A., Yang, J., Vogtenhuber, C., Blazar, B.R. & Farrar, M.A. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290 (2007).
Dang, E.V. et al. Control of TH17/T(reg) balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Sheridan, C. Footrace to clinic heats up for T-cell nuclear receptor inhibitors. Nat. Biotechnol. 31, 370 (2013).
Huang, Z., Xie, H., Wang, R. & Sun, Z. Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin. Ther. Targets 11, 737–743 (2007).
Sherlock, J.P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 18, 1069–1076 2012).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Xie, H., Huang, Z., Wang, R. & Sun, Z. Regulation of thymocyte survival by transcriptional coactivators. Crit. Rev. Immunol. 26, 475–486 (2006).
Xie, H., Sadim, M.S. & Sun, Z. RORγt recruits steroid receptor coactivators to ensure thymocyte survival. J. Immunol. 175, 3800–3809 (2005).
Jetten, A.M., Kurebayashi, S. & Ueda, E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog. Nucleic Acid Res. Mol. Biol. 69, 205–247 (2001).
Holmes, R. & Zuniga-Pflucker, J.C. The OP9–DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harbor Protocols 2009, pdb prot5156 (2009).
Yui, M.A. & Rothenberg, E.V. Developmental gene networks: a triathlon on the course to T cell identity. Nat. Rev. Immunol. 14, 529–545 (2014).
Staschke, K.A. et al. IRAK4 kinase activity is required for Th17 differentiation and Th17-mediated disease. J. Immunol. 183, 568–577 (2009).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Debard, N., Sierro, F. & Kraehenbuhl, J.P. Development of Peyer's patches, follicle-associated epithelium and M cell: lessons from immunodeficient and knockout mice. Semin. Immunol. 11, 183–191 (1999).
van de Pavert, S.A. & Mebius, R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
He, Z. et al. Ubiquitination of RORγt at lysine 446 limits Th17 differentiation by controlling coactivator recruitment. J. Immunol. 197, 1148–1158 (2016).
Miller, M.J., Barrett-Wilt, G.A., Hua, Z. & Vierstra, R.D. Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc. Natl. Acad. Sci. USA 107, 16512–16517 (2010).
Jain, R. et al. Interleukin-23-induced transcription factor Blimp-1 promotes pathogenicity of T helper 17 cells. Immunity 44, 131–142 (2016).
Lazarevic, V. et al. T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 12, 96–104 (2011).
Han, L. et al. The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor γt (RORγt) in Th17 cells. J. Biol. Chem. 289, 25546–25555 (2014).
Wang, X. et al. TRAF5-mediated Lys-63-linked polyubiquitination plays an essential role in positive regulation of RORγt in promoting IL-17A expression. J. Biol. Chem. 290, 29086–29094 (2015).
Yang, J. et al. Cutting edge: Ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORγt. J. Immunol. 194, 4094–4097 (2015).
Okada, S. et al. Immunodeficiencies. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349, 606–613 (2015).
Xie, H., Huang, Z., Sadim, M.S. & Sun, Z. Stabilized β-catenin extends thymocyte survival by up-regulating Bcl-xL. J. Immunol. 175, 7981–7988 (2005).
Ma, J., Wang, R., Fang, X., Ding, Y. & Sun, Z. Critical role of TCF-1 in repression of the IL-17 gene. PLoS One 6, e24768 (2011).
Acknowledgements
We thank J. C. Zuniga-Pflucker (University of Toronto) for the DP9-DL4 stroma cell line; W.S. Pear (University of Pennsylvania) for the retroviral vector MIGR; T. Dawson (Johns Hopkins) for RK5-HA-ubiquitin constructs; and Biocytogen for assisting with design and generation of the RorγtM/M mice. Supported by the US National Institutes of Health (R01-AI053147 and R01-AI109644), institutional pilot funding, the National Cancer Institute of the National Institutes of Health (P30CA33572, which includes work performed in the Animal Resource Center, Integrative Genomics, Analytical Cytometry, and Mass Spectrometry and Proteomics Cores) and the Science and Technology Department of Guangdong Province (2016A050503023 to Z. Huang). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Z. He and Z.S. conceived of the study and wrote the manuscript; Z. He, J.M., R.W., J.Z., Z. Huang, F.W. and S.S. carried out the experiments. E.V.R. instructed and provided critical reagents for in vitro thymocyte development experiments; and all the authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
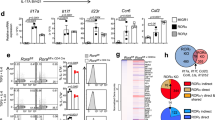
Supplementary Figure 1 RORγt supports in vitro T cell development and TH17 differentiation.
(a) Flow cytometer analyzing the expression of CD24 and TGFβ by CD8+ thymocytes as in Fig.1a. (b) Flow cytometer analysis of CD4 and CD8 in ex vivo development of untransduced GFP- cells from CD4-CD8-RORγt-/- thymocytes transduced with retrovirus expressing various RORγt shown in Fig. 1c. (c) Flow cytometer analysis of various RORγt expression in CD4-CD8-RORγt-/- thymocytes transduced with retrovirus expressing various RORγt and ex vivo development for three days. (d) Flow cytometer analysis of RORγt expression in WT and RORγt-/- CD4+ T cells differentiated under TH17 priming conditions for three days. (e) Flow cytometer analysis of IL-17A+ cells in untransduced GFP- cells from RORγt-/- CD4+ T cells transduced with retrovirus expressing various RORγt as shown in Fig. 1e. (f) Immunoblot analysis of various RORγt expression in cells shown in Fig. 1e. (g) Flow cytometer analysis of ex vivo thymocyte development (top panel) and TH17 differentiation of RORγt-/- cells retrovirally expressing indicated RORγt mutant. (h) Immunoblot of various RORγt expression in RORγt-/- CD4+ T cells transduced with retrovirus expressing indicated RORγt. Full-length gel imagines (f,h), Supplementary Figure 8. Data are from one experiment representative of three experiments (a-h).
Supplementary Figure 2 RORγtM cannot induce genes encoding molecules critical for TH17 differentiation.
(a) Flow cytometer identifying IL-17+ cells among naive RORγt-/- CD4+ T cells retrovirally expressing indicated RORγt polarized under TH17 conditions for three days. (b) Immunoblot analysis of RORγt and RORγt-Flag expression in cells shown in a. (c) ChIP-seq mapped RORγt DNA-binding peaks at Il1r, Il17re, Rorc and Ccr6 loci in RORγt-/- CD4+ T cells retrovirally reconstituted with EV-GFP, RORγt or RORγtM and polarized under TH17 conditions. Full-length gel imagines (b), Supplementary Figure 8. Data are from one experiment representative of three experiments (a,b), or pooled from three experiments (c).
Supplementary Figure 3 Generation of RorγtM/M mice.
(a) Strategy for generation of RORγtM/M mice. To ensure that RORγtM/M is expressed under the control of endogenous RORγt gene locus, a knock-in strategy was used to replace wild type (WT) exon 4 containing the coding sequence for S92L93 without disrupting other DNA sequence. Exon 4 containing S92L93 was replaced by exon with S92A/L93A point mutations via gene targeting vector. A neomycin resistant gene (Neo) for selection of homologous recombination was inserted into the intron between exon 3 and exon 4, so it did not disrupt coding sequence. Neo was also flanked by Frt sites which allow deletion of Neo by flp recombinase (ΔNeo). A diphtheria toxin (DTA) gene cassette was added to select against cells without homologous recombination. (b) PCR analysis of WT allele (left panel), Neo containing allele resulted from recombination (middle panel), and Neo deleted allele (ΔNeo) after crossing with Frt mice (right panel). (c) Sequencing analysis of expression of RORγt with S92A/L93A mutations in thymocytes of RORγtM/M mice. Figures b and c are representative of nine mice.
Supplementary Figure 4 RORγtM supports thymocyte development in vivo.
(a) Flow cytometer analysis of RORγt expression in thymocytes of indicated mice (n=3 per genotype). (b) Flow cytometer analysis of CD44 and CD25 in CD4-CD8-CD3- thymocytes from indicated genotypes of mice (n=5 per genotype). (c) Total number of CD4+, CD8+ and CD4+CD8+ thymocytes averaged from mice of each indicated genotype of mice (n=5). **P < 0.01 (ANOVA). Data are from one experiment representative of three experiments (a,b), or pooled from three experiments (c; plotted as in Fig. 4a).
Supplementary Figure 5 RorγtM/M mice have defective TH17 differentiation.
(a) Flow cytometer analysis of surface CD62L and CD44 on CD4+ and CD8+ T cells from indicated genotypes of mice (n=5 per genotype). (b) Quantification of results shown in c. (c) Flow cytometer analysis of IL-4+ cells among naive CD4+ cells differentiated under TH2 priming conditions for three days. Right panel is the quantification of the results (n=5 per genotype). (d) Flow cytometer analysis of Foxp3+ cells in naive CD4+ cells differentiated under Treg priming conditions for three days. Right panel is the quantification of the results (n=5 per genotype). *P < 0.05 (ANOVA). Data are from one experiment representative of three experiments (a and left panels of c,d), or pooled from three experiments (mean ± s.e.m (in b) or plotted as in Fig. 4a (in right panels of c, d)).
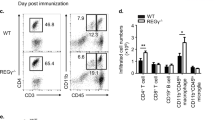
Supplementary Figure 6 RorγtM/M mice are resistant to EAE.
(a and b) Gating strategy for flow cytometric analysis CNS-infiltrated lymphocytes (CD45+) at the peak of the disease after EAE induction in mice described in Fig. 5d (a) and 6b (b). (c) Flow cytometer analysis of indicated cytokines in CD4+ T cells from draining lymph nodes (LN) (top panel) or spleens (bottom panel) of indicated EAE-induced mice described in Fig. 6a (n=5 per genotype). (d) qPCR analysis of Foxp3 mRNA levels in lymphocytes obtained from EAE-induced WT and RORγtM/M mice (n=5 per genotype). (e) Flow cytometer analysis of ILC3 cells in mesenteric lymph nodes (mLN) of indicated genotypes of mice (n=3 per genotype). (f) Number of ILC3 cells from mLN shown in e. Each symbol (d) represents an individual mouse. Data are from one experiment representative of three experiments (a,b,c,e), or pooled from five mice (mean ± s.e.m (in d), or plotted as in Fig. 4a (in f)).
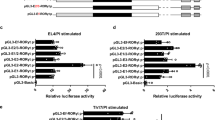
Supplementary Figure 7 RORγtM interferes with the ubiquitination of RORγt at Lys69.
(a) Immunoprecipitation (IP) detection of interaction between various RORγt and SRC1 in HEK 293T cells transfected with indicated expression plasmids. (b) RORγt-luciferase reporter activity in HEK 293T cells transfected with RORγt expression plasmid in the presence (black bars) or absence (open bars) of SRC1 for 24 hrs. (c) Immunoblot of ubiquitinated various RORγt at K69 in HEK 293T cells transfected with expression plasmids for indicated RORγt-K69, together with HA-tagged ubiquitin. RORγt-K69, RORγt have all lysines mutated to arginines except lysine 69. Indicated amino acids, 91-95, 151-155, 156-160, 161-165, were mutated to alanines in RORγt-K69. (d) Flow cytometer analysis of RORγt protein levels in WT and RORγtM/M CD4+ T cells differentiated under TH17 conditions for three days, followed by treatment with protein synthesis inhibitor cycloheximide (CHX) for indicated times. (e) Degradation rate of WT and RORγtM based on data shown in d. (f) Immunoprecipitation (IP) detection of interaction between various RORγt and SRC1 in HEK 293T cells transfected with indicated expression plasmids. ** P < 0.05 (ANOVA). Full-length gel imagines (c,f), Supplementary Figure 8. Data are from one experiment representative of three experiments (a,c,d,f), or pooled from three experiments (b,e; mean ± s.e.m).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1.
Rights and permissions
About this article
Cite this article
He, Z., Ma, J., Wang, R. et al. A two-amino-acid substitution in the transcription factor RORγt disrupts its function in TH17 differentiation but not in thymocyte development. Nat Immunol 18, 1128–1138 (2017). https://doi.org/10.1038/ni.3832
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3832
This article is cited by
-
Extensive transcriptional and chromatin changes underlie astrocyte maturation in vivo and in culture
Nature Communications (2021)
-
5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivative attenuates lupus nephritis with less effect to thymocyte development
Immunologic Research (2021)
-
Sumoylation of RORγt regulates TH17 differentiation and thymocyte development
Nature Communications (2018)
-
Disentangling the manifold functions of RORγt
Nature Immunology (2017)