Abstract
Quantitative and qualitative aspects of nutrition have a profound effect on leukocytes and thereby affect proinflammatory carcinogenic effects or anticancer immune responses. As a result, nutrition affects the incidence, natural progression and therapeutic response of malignant diseases, both in humans and in preclinical animal models. Here we discuss the molecular mechanisms through which alimentary cues modulate metabolic, microbial and neuroendocrine circuitries and thus affect the probability of developing premalignant lesions that progress to clinically manifested disease and the response to therapeutic intervention. We examine each of the connections that compose the triangle of nutrition, immunological and inflammatory reactions and cancer while focusing on the mechanistic aspects of these relationships.
Similar content being viewed by others
Main
People with cancer often rationalize their disease as being a consequence of adverse life experiences; however, large population studies have not established such a correlation1. In sharp contrast, there is overwhelming epidemiological evidence that dietary factors, particularly those that result in overweight and obesity, influence morbidity and mortality in multiple distinct cancers2; however, this connection is often denied at the individual, subjective level. Indeed, the causal link between overnutrition or imbalanced nutrition and cancer is obfuscated for multiple reasons.
First, at the population level, overnutrition and imbalanced diets are typically associated with other health-compromising factors that include, but are not limited to, consumption of tobacco and alcohol, decreased physical activity and exposure to environmental toxicants, all of which not only are established risk factors for cancer development but also are linked to poor education and low income. Low socioeconomic status is yet another independent risk factor for premature mortality, including death from cancer3. Hence, it is intrinsically difficult to 'isolate' dietary risk factors in epidemiological studies. That being said, careful experimentation in rodents has irrefutably demonstrated the influence of nutrition on cancer development4.
Second, in contrast to continuous variables, such as blood pressure or glucose concentrations, cancer occurs as an all-or-nothing phenomenon, as the clinical manifestation of stochastic events influenced by the number of stem cell divisions5 and a debatable degree of 'bad luck' due to hazardous mutations, as well as avoidable causes associated with lifestyle factors6. Undoubtedly, the most important intrinsic risk factor in cancer is old age, thus suggesting that measures that slow down the aging process, such as caloric restriction, or that accelerate aging, such as overnutrition7, have large effects on the incidence of cancer; without these effects, there might be a direct cause-and-effect relationship between unhealthful behavior and carcinogenesis. Unsurprisingly, common risk factors determine the incidence of neoplastic and cardiovascular diseases8. Hence, many effects of nutrition on the development and progression of cancer lack 'specificity' in their association, and such alimentary cues also affect the risk of developing metabolic syndrome, a prelude to arteriosclerosis and neurodegeneration (Fig. 1).
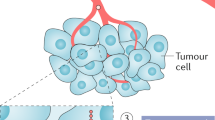
The triangle composed of nutrition, inflammation and immunity, and cancer illustrates how qualitative and quantitative imbalances in food intake predispose organisms, in a manner dependent on or independent of the immune system, to a time-dependent deterioration in function that culminates in the development and progression of cancer. Image adapted from Servier Medical Art (http://www.servier.com/Powerpoint-image-bank/) under the terms of the CC BY 3.0 license.
Third, the relationship between diet and cancer is not as linear as those between UV light and melanoma, or between tobacco use and lung cancer (perhaps with the exception of orally available chemical mutagens in tobacco that directly cause gastrointestinal cancers9, which will not be discussed here). In general, nutrition affects cancer development somewhat indirectly. For example, the microbiome can be affected by changes in carcinogenic or tumor-suppressive metabolites and microbe-associated molecular patterns generated in the gut10; endocrine factors, such as concentrations of circulating insulin, insulin-like growth factor, leptins and adiponectins2; and inflammatory and immunological parameters, which are interrelated because the same leukocyte populations contribute to both inflammation and immune responses.
In this Review, we focus on the complex relationships among nutrition, inflammation–immunity and cancer. We examine each side of this triangle separately (Fig. 1) and provide a unifying hypothesis of how nutritional imbalances may affect the development and progression of cancer.
Inflammation, immunity and cancer
Although the relationship between chronic inflammation and cancer ('the wound that never heals') has long been acknowledged11, the idea that cancer is under immunosurveillance has only recently gained broad acceptance12. One conceptual difficulty that has been (and remains) difficult to resolve is that many of the leukocyte subsets and factors that govern inflammation also influence immune reactions, and vice versa. For example, inflammasomes serve a dual role in inflammation and immunity and thus favor or suppress tumorigenesis in a highly context-dependent fashion. The ambivalent contribution of inflammasomes to carcinogenesis and immunosurveillance is most probably based on the existence of a variety of distinct inflammasome complexes and inflammasome products that are expressed differentially in tissues and cell types in an activation-state-dependent manner12,13,14.
Chronic inflammation is carcinogenic, as exemplified by reflux esophagitis, gastritis, inflammatory bowel disease, silicosis-associated lung cancer, asbestosis-induced mesothelioma and various types of hepatitis induced by viruses, alcohol consumption or a Western-style diet11. The carcinogenic effects of inflammation may be dependent on chronic stimulation of cellular turnover, with a consequent increase in stem-cell divisions, as well as on local mutagenic effects. Mutagenesis may be promoted by the inflammation-causing agent itself and by enhanced production of reactive oxygen species that either are a side product of locally enhanced metabolism or are generated specifically by macrophages15. In addition, unresolved inflammation favors trophic interactions between parenchymatous cells and infiltrating leukocytes as it antagonizes immunosurveillance. As an example, alternatively activated (M2) macrophages and granulocytes can act as myeloid-derived suppressor cells within incipient tumor lesions16. Similarly, the cytokine TGF-β, which has a major role in fibrotic reactions, is also a major local immunosuppressor17.
The relationship between neoplasia and the immune system has been didactically condensed into the '3E hypothesis', which involves initial elimination of transformed cells by immunological effector cells; subsequent equilibrium between malignant cells and the immune reaction within a smoldering (and mostly indolent) neoplastic lesion, and final escape of cancer cells from immunological control18. That final event, which is linked to clinical disease manifestation, often after a long latency, can involve the selection of nonimmunogenic cancer cells (immunoediting) or active suppression of the local immune response (immunosubversion)18,19. Successful treatment of cancer with chemotherapy or radiotherapy reestablishes the equilibrium state by reactivating immunosurveillance, usually by increasing the immunogenicity of cancer cells, which release danger-associated molecular patterns, and/or by depleting immunosuppressive leukocytes such as myeloid-derived suppressor cells and regulatory T cells from the tumor bed20,21. Similarly, immunotherapy with immunological-checkpoint blockers reactivates anticancer responses mediated by exhausted T cells, provided that such cells are already present in the tumor immune-cell infiltrate22,23.
Distinguishing between protumorigenic effector cells and anticancer effector cells in the local leukocyte infiltrate requires accurate phenotyping of various cell types (to distinguish myeloid cells with differing polarities as well as distinct T lymphocyte populations that may have regulatory and effector functions) through multicolor immunohistochemistry, cytofluorometric analysis of monocellular suspensions24 or single-cell transcriptomics25. The single-cell-transcriptomics approach has an advantage in that it reveals the T cell repertoire of infiltrating T lymphocytes and thus yields clues as to their possible specificities. Additional techniques might involve the ex vivo culture of tumor slices26 or oligocellular preparations, the addition of immunostimulators or checkpoint blockers, and final quantification of cytokines, T cell–activation markers or immune-cell trajectories27.
Nutrition, inflammation and immunity
Awareness that nutrition influences inflammatory and immune responses has led to the development of the 'dietary inflammatory index'28 and the use of terms such as 'immunonutrition'29 in epidemiological and clinical studies. Nonetheless, the effects of individual macro- and micronutrients on leukocyte function have not been studied in great molecular detail, perhaps with the exception of hypovitaminoses. At present, strong immunomodulatory effects have been revealed for quantitative variations in nutrient uptake, particularly in overeating and fasting (Fig. 2).
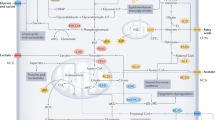
Genetics or lifestyle-driven changes in body composition (e.g., overfeeding or a Western-style diet) result in a prepathological phenotype (metabolic syndrome) or pathological phenotype (obesity); these phenotypes together accelerate cellular transformation, aging and resistance to anticancer treatments, through multiple mechanisms. Elevated nutrient intake favors the generation of a subclinical inflammatory status with a consequent increase in circulating proinflammatory cytokines; determines the excessive increase in circulating glucose and lipid concentrations; and increases the levels of trophic hormones. Together those alterations produce a vicious cycle that leads to inhibition of autophagy and satisfies the metabolic needs of cancer cells. In addition, stromal cancer-associated adipocytes (CAAs) feed that circuitry and support tumor progression. Alternatively, an excess of nutrients can subvert immunological function, either directly or indirectly, through modification of the composition and metabolism of the intestinal microbiota. Such aberrations render the tumor microenvironment immunosuppressive and impair the therapeutic response to anticancer agents. CHI3L1, chitinase 3-like 1; SPP1, secreted phosphoprotein 1; SERPINE1, Serpin family E member 1. Image adapted from OpenClipart (https://openclipart.org/) under the terms of the CC0 1.0 license.
Hyperadiposity is linked to chronic subclinical inflammation through a variety of mechanisms30. Obesity is associated with a switch in the phenotype of adipose-tissue macrophages from the M2 anti-inflammatory phenotype to a so-called 'M1 proinflammatory' phenotype; enhanced production of multiple proinflammatory cytokines, such as TNF and the interleukins IL-6 and IL-1β; an elevation in glucose and free fatty acids, leptin, insulin and the insulin-like growth factor IGF1; a decrease in adiponectin; and intestinal dysbiosis with diminished barrier function, which favors endotoxinemia and overrepresentation of bacterial species producing procarcinogenic metabolites2,31. Saturated free fatty acids promote inflammation by binding to the Toll-like receptors TLR4 and TLR2 through the adaptor protein fetuin 1 and consequently activating proinflammatory factors such as NF-κB and JNK1 (ref. 32). Obesity is also linked to systemic inhibition of autophagy as a result of high intracellular concentrations of acetyl coenzyme A33,34, elevated trophic signaling that results in activation of the metabolic checkpoint kinase mTOR and, in some organs such as the liver, downregulation of the proautophagic ATG7 protein35. The subversion of autophagy results in a decrease in the cytoplasmic recycling of damaged organelles, with consequent acceleration of the aging process7, accumulation of potentially oncogenic products such as SQSTM1 (best known as p62)36 and uninhibited activation of the NLRP3 inflammasome37. Interestingly, a high-fat diet stimulates generation of the complement fragment C5a, even in mouse strains that are resistant to obesity induced by such a diet, and this sign of complement activation has been causally linked to high-fat-diet-triggered intestinal inflammation and accelerated carcinogenesis in ApcMin/+ mice, a genetic model of spontaneous intestinal tumorigenesis38.
Obese people exhibit many immunological abnormalities also associated with old age39, in line with the proposal that obesity accelerates aging7. Thus, obese people have fewer cytotoxic CD8+ T cells40, perhaps as a consequence of hastened thymic atrophy41 or diminished dendritic-cell function42. Moreover, obesity is associated with a decreased number and function of natural killer cells, as well as with a substantial decrease in circulating Vγ9+Vδ2+ T cells43. Experimental anticancer immunotherapies that work in lean mice might fail in44 or be toxic to45 obese mice. Thus, the lethal cytokine storm that manifests in obese mice after immunostimulation with antibody to the costimulatory receptor CD40 and with CpG dinucleotides has been attributed to enhanced production of TNF by adipose-tissue macrophages45. Together, these findings support the importance of considering information about body weight during the clinical development of immuno-oncology drugs.
Fasting has considerable anti-inflammatory effects that are clinically exploited in some countries, such as for the treatment of rheumatoid arthritis. A 24-hour period of fasting in vivo has been shown to decrease the in vitro lipopolysaccharide-induced NLRP3-inflammasome-dependent production of IL-1β by monocytes from human volunteers46. This effect has been linked to activation of the energy biosensor sirtuin-3, a deacetylase. Notably, the starvation-induced inhibition of IL-1β production is lost only 3 hours after refeeding of a hypocaloric (500-kcal) meal46, thus suggesting that such inhibition is highly sensitive to even small variations in nutrient uptake. Fasting also causes protein hypoacetylation and signs of autophagy in circulating peripheral blood mononuclear cells from human volunteers47; hence, the observed inhibition of NLRP3 inflammasomes might be related to autophagy induction.
In mice, fasting increases resistance to infection with Listeria monocytogenes or to injection of the TLR4 ligand lipopolysaccharide. This beneficial effect of fasting, which is subverted by intraperitoneal injection of glucose and can be mimicked by therapeutic blockade of glucose use via 2-deoxy-D-glucose, has been explained by an increase in ketone bodies (described below). However, this effect is not linked to a decrease in the production of proinflammatory factors such as IL-6 and TNF48. In contrast, fasting or treatment with 2-deoxy-D-glucose sensitizes mice to infection with vaccinia virus or to injection of the synthetic RNA duplex poly(I:C)), a ligand of TLR3, thereby resulting in increased death48.
Thus, the feeding state appears to have a substantial effect on inflammatory and immune responses. Indeed, even in lean mice, feeding causes a physiological increase in circulating IL-1β that has been attributed to the action of the microbiome and increased glucose concentrations in peritoneal macrophages and that might contribute to postprandial stimulation of insulin secretion49. However, beyond physiology, spontaneous changes in feeding behavior—such as sickness-associated anorexia, which is often triggered by infections or severe disease—might be an evolutionarily conserved defense strategy for decreasing excessive inflammation and improving immune responses, such as by increasing autophagic flux50 (Fig. 3).
Fasting or a fasting-mimicking diet (FMD) and caloric-restriction mimetics (CRMs) elicit an anticancer immune response through convergent mechanisms. A decrease in circulating concentrations of IGF1 and the activation of autophagy promote an increase in precursors of CD8+ T lymphocytes, mediated by heme oxygenase 1 (HMOX-1), in the bone marrow (BM) and less release of immunostimulatory ATP from tumor cells. When combined with cytotoxic chemotherapeutic agents, fasting and caloric-restriction mimetics modify the tumor immune-cell infiltrate. Such changes include an increase in infiltrating cytotoxic CD8+ T lymphocytes and antigen-presenting myeloid cells and a decrease in immunosuppressive Foxp3+ regulatory T cells. In addition, an increase in circulating ketone bodies elicited by fasting or a fasting-mimicking diet participates in degradation of the NRLP3 inflammasome, an effect that can be replicated by the activation of autophagy. Together these strategies limit inflammatory reactions and enhance the adaptive immune response to tumor cells.
A high-fat, adequate-protein, low-carbohydrate diet causes a decrease in circulating glucose concentrations together with an increase in free fatty acids and ketone bodies, such as acetoacetate and β-hydroxybutyrate. Collectively, these changes also occur after fasting or exercise51. The ketone body β-hydroxybutyrate is highly efficient in inhibiting the NLRP3 inflammasome, but not the NLRC4 inflammasome, by preventing cytosolic potassium influx and consequent formation of aggregates consisting of the adaptor ASC and the NLRP3 inflammasome52. Notably, the beneficial effects of fasting on bacterial infection and lipopolysaccharide-mediated toxic shock are mediated by ketone bodies48, although it has not been determined whether these effects involve inhibition of the NLRP3 inflammasome with a consequent decrease in IL-1β. Beyond its effects on the NLRP3 inflammasome, β-hydroxybutyrate is an endogenous ligand of the G-protein-coupled receptor GPR109A (HCAR2), which is abundantly expressed by monocytes and macrophages and has anti-inflammatory effects51. Hence, ketone bodies dampen inflammation through several mechanisms (Fig. 3).
Nutrition and cancer epidemiology
Obesity will probably become the most important avoidable risk factor for malignancy, before tobacco, and it has been demonstrated to affect the incidence and progression of various solid cancers53. Obesity concurrent with metabolic syndrome appears to be more closely associated with cancer than is obesity concurrent with 'metabolic health'54. Hence, distinguishing different types of obesity, rather than focusing exclusively on the body mass index, appears to be crucial, given that central obesity with 'preferential' accumulation of visceral fat and a consequent increase in waist circumference is associated with the highest incidence of metabolic syndrome. Metabolic syndrome–associated factors, such as high blood pressure and circulating C-reactive protein, glucose, IL-6, insulin, leptin and triglycerides—in contrast to low adiponectin and high-density-lipoprotein cholesterol—are associated with the presence of macrophages that form crown-like structures in the breast tissue, which are considered to be a sign of white-adipose-tissue inflammation and a marker of poor prognosis55. In mice, obesity-driven breast-cancer progression requires the NLRC4 inflammasome, and expression of NLRC4 mRNA in noncancerous human breast tissue indeed correlates with body mass index and is high in mammary carcinomas56. Such findings underscore the strong relationship between metabolism and procarcinogenic inflammation.
Beyond the unquestionable links among quantitative overnutrition, inflammation and elevated cancer risk, epidemiological studies have linked cancer to qualitative disequilibria in food composition that have been tentatively condensed into unidimensional numeric values. Thus, the 'dietary inflammatory index' weights each major macronutrient and multiple micronutrients on the basis of their general proinflammatory effects, as measured, for example, by assessment of C-reactive protein in serum28. This index significantly correlates with the risk of developing postmenopausal breast cancer57, colorectal cancer58 or lung cancer in smokers59, among other examples. Alternative scores, such as the 'Mediterranean diet score' and the 'healthy eating index', have also been associated with the risk of developing colorectal cancer60.
Micronutrients with anticancer properties include some vitamins. For example, high dietary intake of dietary vitamin B6 (pyridoxine) and high plasma concentrations of its metabolite, PLP (pyridoxal-5′-phosphate), are associated with a general decrease in cancer risk, particularly in gastrointestinal cancers61. In mouse models, pyridoxine stimulates the anticancer immune response in the context of cisplatin-based chemotherapy62. Moreover, high expression of pyridoxine kinase, the enzyme that converts pyridoxine into PLP, is associated with favorable prognosis in non-small-cell lung cancer63. High concentrations of 25-hydroxyvitamin D in serum are associated with favorable prognosis in patients diagnosed with breast cancer64, prostate cancer65 or colorectal cancer66. High concentrations of 25-hydroxyvitamin D in plasma are also correlated with a decreased frequency of colorectal cancers with little lymphocyte infiltration, which are associated with a particularly poor prognosis67. Furthermore, elevated concentrations of 25-hydroxyvitamin D in plasma are correlated with decreased proinflammatory markers and favorable prognosis in people with prostate cancer68,69. These results are reminiscent of those obtained for another well-established cancer-preventive agent, aspirin, which also decreases the incidence of colorectal cancers with low frequencies of tumor-infiltrating lymphocytes70. Moreover, these findings are similar to those of mouse experiments showing that knockout of the gene encoding the vitamin D receptor precludes the maturation and proliferation of intestinal CD8αα+ intraepithelial lymphocytes71 and accelerates the development of colorectal carcinoma in cancer-prone ApcMin/+ mice72. Supplementation with 1,25-dihydroxyvitamin D3 plus calcium in healthy volunteers has been found to induce measurable expression of immune-system-related genes in rectosigmoid colonic mucosa. Together, these findings support the idea that specific micronutrients stimulate anticancer immunosurveillance73 (Fig. 4).
Diet-derived vitamin B6 and vitamin D3 are inactive in their natural form. After being passively transported into cells, these molecules are converted intracellularly into metabolites that are responsible for the consequent biological effects. Within tumor cells, pyridoxine kinase (PDXK)-mediated phosphorylation of vitamin B6 results in the generation of its metabolite, PLP. By increasing endoplasmic-reticulum (ER) stress, PLP elicits immunogenic-cell-death features in cancer cells and hence enhances the antineoplastic activity of cisplatin (CCDP) in an immune-system-dependent fashion. Vitamin D3 undergoes hydroxylation reactions mediated by the cytochrome p450 enzymes CYP27A1 and CYP27B1 and is converted into bioactive 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in hepatic, renal and immune cells. Through binding to nuclear vitamin D receptor (VDR), 1,25(OH)2D3 promotes an anti-inflammatory and immunostimulatory gene-expression signature responsible for its cancer-preventive action. 25(OH)D3, 25-hydroxyvitamin D3.
In addition to fiber, multiple distinct phytochemicals have been found to prevent colon-cancer development in mouse models, in a manner associated with effects on the gut microbiome and anti-inflammatory effects10. Serum concentrations of enterolactone, which reflect the uptake of dietary lignans, positively correlate with overall survival in women with postmenopausal breast cancer74, and flaxseed lignin administered to postmenopausal women decreases the concentration of C-reactive protein75. The uptake of dietary spermidine, which is particularly abundant in some fruits, soybean sprouts and mature cheeses, has also been linked to an overall decrease in human cancer mortality76. In mice, spermidine supplementation decreases subclinical inflammation76 and stimulates anticancer immune responses77.
Coffee consumption has a prominent anti-inflammatory effect, as indicated by epidemiological studies linking the abundance of caffeine metabolites in the blood to the expression of inflammasome-related genes, the amounts of NLRC4-inflammasome-activating metabolites, such as adenine and N4-acetylcytidine and circulating IL-1β (ref. 78). Indeed, caffeine inhibits the secretion of IL-1β induced by adenine and N4-acetylcytidine78. Independently of its caffeine content, coffee also induces autophagy in vivo, in mice79. Whereas coffee consumption causes a dose-dependent decrease in overall and cardiovascular mortality, it does not affect cancer mortality, with the notable exception of hepatocellular carcinoma80 and perhaps colorectal cancer10,81.
The consumption of nuts is also inversely associated with death due to cancer or cardiovascular disease82,83. Indeed, nut intake is associated with a diminished subclinical inflammatory status, as reflected by the plasma concentrations of C-reactive protein83. This effect might be related to the high content of omega-3 fatty acids in nuts, which are known for their health-promoting and anti-inflammatory effects84, although this possibility requires further in-depth investigation.
The mechanisms that account for the main protumorigenic effects of red-meat consumption on cancer risk in multiple organs, such as the colon, prostate and breast, remain to be resolved81. One intriguing but unproven hypothesis posits that incorporation of an alien metabolite, N-glucolylneuraminic acid, which is particularly abundant in beef, into the tissues of red-meat consumers might lead to the proinflammatory induction of xenoantigen-specific antibodies81. Collectively, there is abundant but fragmentary evidence relating nutrition to the development and progression of cancer. In several cases, these links causally involve immunomodulatory effects.
Nutritional interventions for cancer treatment
For many decades, nutritional interventions have been conceived mainly to treat the wasting syndrome cachexia in terminally ill cancer patients. Cachexia is often caused by proinflammatory factors, such as TNF and IL-6 produced by tumor cells, and changes in systemic metabolism; it is characterized by systemic immunosuppression that may be due to elevated glucocorticoid tonus85. Here we discuss nutritional interventions for the treatment of cancer in a curative setting rather than a palliative setting.
Bariatric surgery decreases cancer mortality86; this is at odds with the observation that lean, formerly obese mice, in contrast to mice that have never been obese, carry an epigenetic memory that causes accelerated growth of transplantable mammary carcinomas87. In mouse models bearing a series of distinct transplantable cancers, treatment with one or several fasting cycles (usually for 48 hours, thus causing a transient but drastic decrease of ∼20% in body weight) diminishes tumor growth, especially if the treatment is combined with chemotherapy88. Notably, most of the beneficial effects of fasting require an intact cellular immune system, given that tumors implanted into nude mice, which lack T lymphocytes, do not result in decreased tumor growth after treatment with anthracyclines or oxaliplatin together with starvation89. Instead of starvation, a hypocaloric diet can be used to boost chemotherapy-induced anticancer immunosurveillance, with an increase in circulating and tumor-infiltrating CD8+ T cells, local depletion of regulatory T cells and a surge in common lymphoid precursor cells in the bone marrow89. Such effects have been linked to the upregulation of autophagy and the downregulation of Nrf2 and HO-1, proteins involved in anti-inflammatory responses in cancer cells77,89 (Fig. 3). In addition, a hypocaloric diet has been shown to promote the regeneration of insulin-secreting pancreatic beta cells and thus to correct diet-driven metabolic syndromes90. Given these insights, fasting or hypocaloric diets might also prevent cellular transformation by maintaining the stemness of tissue-specific progenitor cells91.
Starvation causes a rapid decrease in protein acetylation both in mouse tissues33,77 and in peripheral blood mononuclear cells from human volunteers47. Protein deacetylation is a potent stimulus of autophagy and can be artificially induced by a series of caloric-restriction mimetics, which can be grouped into three categories: (i) agents, such as hydroxycitrate, that decrease the synthesis of cytosolic acetyl coenzyme A; (ii) agents, such as spermidine, that inhibit acetyltransferases such as EP300; and (iii) agents, such as resveratrol, that activate deacetylases such as sirtuin-1 (ref. 92). Such agents, many of which are part of a normal diet, induce autophagy without causing immunosuppression and can be used to stimulate anticancer immune responses elicited by chemotherapy. This effect requires the induction of autophagy in cancer cells; activates an increase in the extracellular release of ATP from stressed tumor cells in vivo; favors remodeling of the immune-cell infiltrate with an increased recruitment of dendritic-cell precursors, for which ATP is a chemotactic factor; and improves the CD8+ cell/Foxp3+ cell ratio77. Similarly to starvation, treatment with caloric-restriction mimetics decreases free concentrations of the insulin-like growth factor IGF1. Replenishing IGF1 blocks autophagy induced by caloric-restriction mimetics and abolishes their anticancer effects77. Hence, the immunostimulatory effects of starvation and caloric-restriction mimetics overlap in their modes of action (Fig. 3). Notably, rapamycin, another drug that induces autophagy and increases longevity in model organisms, cannot be considered a typical caloric-restriction mimetic, because it does not decrease acetylation of cytoplasmic proteins33,92.
Although they vary in their anticancer effects, ketogenic diets might prolong survival in animal models and human studies, as suggested by meta analyses, primarily in brain neoplasia93,94. Mice with intracranial glioblastomas exhibit improved tumor-reactive innate and adaptive immune responses, including increased cytokine production and cytolysis of tumor cells by specific CD8+ T cells, after treatment with a ketogenic diet. This effect is coupled to a decrease in markers of T cell exhaustion, such as PD-1 and CTLA-4 (ref. 95). Similarly, a ketogenic diet inhibits lactate production by glycolytic tumors, an effect secondary to a decrease in circulating glucose concentrations, and thereby reduces lactate-mediated local immunosuppression, which in turn decreases the frequency of myeloid-derived suppressor cells and improves anticancer immune responses96 (Fig. 3).
Given the epidemiological evidence discussed above, it appears plausible to attempt dietary interventions or to provide food supplements that increase the uptake of spermidine and vitamins B6 and D (Fig. 4), although clinical trials using vitamin E and beta carotene have been unsuccessful or even have been associated with increased cancer incidence97. Vitamin B3 (nicotinamide) has been found in a phase III clinical trial to significantly decrease the incidence of nonmelanoma skin cancers, but its mode of action has not been determined98. This effect might be part of the general antiaging effect of nicotinamide99, and evaluation of its chemopreventive or therapeutic action might be extended to other malignancies. In the course of such trials, it will be important to assess immunological parameters that might prove to be useful biomarkers and yield heuristic insights.
Concluding remarks and outlook
The premises noted above allow several overarching conclusions to be drawn. First, there is compelling evidence that nutrition has considerable effects on the incidence and progression of cancer and responses to treatment. Second, a lifestyle characterized by caloric excess, sedentarism and a high-fat, high-sugar Western-style diet tends to promote carcinogenesis through diverse intertwined mechanisms that include, but are not limited to, increased inflammatory reactions; diminished immunosurveillance; and a considerable abundance of energy-rich metabolites (such as glucose, fatty acids or amino acids) or trophic factors (such as insulin, insulin-like growth factor and leptin) that might stimulate the multiplication of malignant cells. Corrective interventions regarding the quantity and quality of nutrition have the potential to prevent cancer or slow its progression. Moreover, targeted interventions with defined macro- and micronutrients can dampen procarcinogenic inflammation and stimulate anticancer immunosurveillance.
Unfortunately, most past cancer research has been based on the assumption that the growth of malignant cells in vitro or in immunodeficient mice reflects in vivo physiology, and the effects of metabolic factors on cancer growth have usually been studied with this perspective. Future work will need to integrate the fundamental effects of an intact immune system on the evolution of tumors. Doing so would require the use of holistic experimental approaches in which the effects of nutrition on cancer are studied in fully immunocompetent mice with a 'humanized' microbiome. Moreover, future clinical trials in this area must incorporate sophisticated immunomonitoring approaches to yield interpretable results.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Schoemaker, M.J. et al. Psychological stress, adverse life events and breast cancer incidence: a cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Res. 18, 72 (2016).
Font-Burgada, J., Sun, B. & Karin, M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 23, 48–62 (2016). This paper, from major researchers in the field, provides an excellent overview of the link between obesity and cancer.
Stringhini, S. et al. Socioeconomic status and the 25×25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 389, 1229–1237 (2017).
Deng, T., Lyon, C.J., Bergin, S., Caligiuri, M.A. & Hsueh, W.A. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 11, 421–449 (2016).
Tomasetti, C. & Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015).
Tomasetti, C., Li, L. & Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 355, 1330–1334 (2017).
López-Otín, C., Galluzzi, L., Freije, J.M., Madeo, F. & Kroemer, G. Metabolic control of longevity. Cell 166, 802–821 (2016). This review summarizes the cause-and-effect relationship between the metabolic features of Western-style diets and an increased probability of developing life-threating cancer.
Koene, R.J., Prizment, A.E., Blaes, A. & Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 133, 1104–1114 (2016).
Hullar, M.A., Burnett-Hartman, A.N. & Lampe, J.W. Gut microbes, diet, and cancer. Cancer Treat. Res. 159, 377–399 (2014).
O'Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 13, 691–706 (2016).
Schäfer, M. & Werner, S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9, 628–638 (2008).
Palucka, A.K. & Coussens, L.M. The basis of oncoimmunology. Cell 164, 1233–1247 (2016).This article provides an overview of the major concepts and successes in tumor immunology and anticancer immunotherapy.
Zitvogel, L., Kepp, O., Galluzzi, L. & Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 13, 343–351 (2012).
Karki, R., Man, S.M. & Kanneganti, T.D. Inflammasomes and cancer. Cancer Immunol. Res. 5, 94–99 (2017).
Nathan, C. & Cunningham-Bussel, A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 (2013).
Elliott, L.A., Doherty, G.A., Sheahan, K. & Ryan, E.J. Human tumor-infiltrating myeloid cells: phenotypic and functional diversity. Front. Immunol. 8, 86 (2017).
Chen, W. & Ten Dijke, P. Immunoregulation by members of the TGFβ superfamily. Nat. Rev. Immunol. 16, 723–740 (2016).
Mittal, D., Gubin, M.M., Schreiber, R.D. & Smyth, M.J. New insights into cancer immunoediting and its three component phases: elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).This paper provides an excellent synthesis of the major rules governing the relationship between oncogenesis and anticancer immunosurveillance.
Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6, 715–727 (2006).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Krysko, D.V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Sharma, P. & Allison, J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Pitt, J.M. et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269 (2016).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Bougherara, H. et al. Real-time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front. Immunol. 6, 500 (2015).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015). This paper provides genetic evidence supporting the idea that the success of anticancer chemotherapies depends on the immune system, in human breast cancer and colorectal carcinoma.
Shivappa, N., Steck, S.E., Hurley, T.G., Hussey, J.R. & Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17, 1689–1696 (2014).
Prieto, I., Montemuiño, S., Luna, J., de Torres, M.V. & Amaya, E. The role of immunonutritional support in cancer treatment: current evidence. Clin. Nutr. http://dx.doi.org/0.1016/j.clnu.2016.11.015 (2016).
Iyengar, N.M., Gucalp, A., Dannenberg, A.J. & Hudis, C.A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J. Clin. Oncol. 34, 4270–4276 (2016).
Louis, P., Hold, G.L. & Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672 (2014).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Mariño, G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell 53, 710–725 (2014).
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J.M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
Yang, L., Li, P., Fu, S., Calay, E.S. & Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Umemura, A. et al. p62, upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell 29, 935–948 (2016).
Zhong, Z., Sanchez-Lopez, E. & Karin, M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166, 288–298 (2016).
Doerner, S.K. et al. High-fat diet-induced complement activation mediates intestinal inflammation and neoplasia, independent of obesity. Mol. Cancer Res. 14, 953–965 (2016). This paper formally demonstrates that a high-fat diet can trigger intestinal carcinogenesis even in mice genetically resistant to the induction of obesity.
Klevorn, L.E. & Teague, R.M. Adapting cancer immunotherapy models for the real world. Trends Immunol. 37, 354–363 (2016). This important conceptual paper investigates the multiple modulatory factors (including obesity) that affect the efficacy of the immune system, with regard to the recognition of neoplastic cells in the context of immunotherapies.
Lamas, O., Marti, A. & Martínez, J.A. Obesity and immunocompetence. Eur. J. Clin. Nutr. 56 (Suppl. 3), S42–S45 (2002).
Yang, H. et al. Obesity accelerates thymic aging. Blood 114, 3803–3812 (2009).
Macia, L. et al. Impairment of dendritic cell functionality and steady-state number in obese mice. J. Immunol. 177, 5997–6006 (2006).
Conroy, M.J., Dunne, M.R., Donohoe, C.L. & Reynolds, J.V. Obesity-associated cancer: an immunological perspective. Proc. Nutr. Soc. 75, 125–138 (2016).
James, B.R. et al. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J. Immunol. 189, 1311–1321 (2012).
Mirsoian, A. et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J. Exp. Med. 211, 2373–2383 (2014). This paper shows that preexisting adiposity limits the efficacy of immunotherapy in an age-dependent manner by inducing the production of proinflammatory and cytotoxic cytokines.
Traba, J. et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J. Clin. Invest. 125, 4592–4600 (2015). This pilot study demonstrates the major effects of short-term fasting on the inflammatory response.
Pietrocola, F. et al. Metabolic effects of fasting on human and mouse blood in vivo. Autophagy 13, 567–578 (2017).
Wang, A., Huen, S.C., Luan, H.H., Yu, S., Zhang, C. & Gallezot, J.D. et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525.e1512 (2016). This paper investigates voluntary fasting as a physiological component of the sickness response elicited by bacterial infection.
Dror, E. et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 18, 283–292 (2017).
van Niekerk, G., Loos, B., Nell, T. & Engelbrecht, A.M. Autophagy: a free meal in sickness-associated anorexia. Autophagy 12, 727–734 (2016).
Puchalska, P. & Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Youm, Y.H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015). This paper demonstrates that fasting-induced production of β-hydroxybutyrate limits inflammation by hindering NRLP3 inflammasome activation.
Hopkins, B.D., Goncalves, M.D. & Cantley, L.C. Obesity and cancer mechanisms: cancer metabolism. J. Clin. Oncol. 34, 4277–4283 (2016). This excellent position paper describes the major negative effects of obesity on oncogenesis and cancer progression.
Moore, L.L., Chadid, S., Singer, M.R., Kreger, B.E. & Denis, G.V. Metabolic health reduces risk of obesity-related cancer in Framingham Study adults. Cancer Epidemiol. Biomarkers Prev. 23, 2057–2065 (2014).
Iyengar, N.M. et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 22, 2283–2289 (2016).
Kolb, R. et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat. Commun. 7, 13007 (2016).
Shivappa, N., Blair, C.K., Prizment, A.E., Jacobs, D.R. & Hebert, J.R. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol. Nutr. Food Res. 61, 1600592 (2016).
Harmon, B.E. et al. The dietary inflammatory index is associated with colorectal cancer risk in the multiethnic cohort. J. Nutr. 147, 430–438 (2017).
Hodge, A.M. et al. Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control 27, 907–917 (2016).
Steck, S.E., Guinter, M., Zheng, J. & Thomson, C.A. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv. Nutr. 6, 763–773 (2015).
Mocellin, S., Briarava, M. & Pilati, P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J. Natl. Cancer Inst. 109, 1–9 (2017).
Aranda, F. et al. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene 34, 3053–3062 (2015).
Galluzzi, L. et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2, 257–269 (2012).
Goodwin, P.J., Ennis, M., Pritchard, K.I., Koo, J. & Hood, N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J. Clin. Oncol. 27, 3757–3763 (2009).
Tretli, S., Hernes, E., Berg, J.P., Hestvik, U.E. & Robsahm, T.E. Association between serum 25(OH)D and death from prostate cancer. Br. J. Cancer 100, 450–454 (2009).
Dou, R. et al. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br. J. Nutr. 115, 1643–1660 (2016).
Song, M. et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut 65, 296–304 (2016). This work provides the first evidence of an inverse correlation between levels of 25-hydroxyvitamin D and colorectal cancer risk, according to the degree of lymphocyte infiltration in the tumor bed.
Giangreco, A.A. et al. Differential expression and regulation of vitamin D hydroxylases and inflammatory genes in prostate stroma and epithelium by 1,25-dihydroxyvitamin D in men with prostate cancer and an in vitro model. J. Steroid Biochem. Mol. Biol. 148, 156–165 (2015).
Batai, K., Murphy, A.B., Nonn, L. & Kittles, R.A. Vitamin D and immune response: implications for prostate cancer in African Americans. Front. Immunol. 7, 53 (2016).
Cao, Y. et al. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology 151, 879–892.e4 (2016).
Bruce, D. & Cantorna, M.T. Intrinsic requirement for the vitamin D receptor in the development of CD8αα-expressing T cells. J. Immunol. 186, 2819–2825 (2011).
Larriba, M.J. et al. Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer. PLoS One 6, e23524 (2011).
Protiva, P. et al. Calcium and 1,25-dihydroxyvitamin D3 modulate genes of immune and inflammatory pathways in the human colon: a human crossover trial. Am. J. Clin. Nutr. 103, 1224–1231 (2016).
Buck, K. et al. Serum enterolactone and prognosis of postmenopausal breast cancer. J. Clin. Oncol. 29, 3730–3738 (2011).
Hallund, J., Tetens, I., Bügel, S., Tholstrup, T. & Bruun, J.M. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr. Metab. Cardiovasc. Dis. 18, 497–502 (2008).
Eisenberg, T. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438 (2016).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016). This paper demonstrates that caloric-restriction mimetics are as efficient as fasting in stimulating an autophagy-dependent anticancer immune response.
Furman, D. et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 23, 174–184 (2017).
Pietrocola, F. et al. Coffee induces autophagy in vivo. Cell Cycle 13, 1987–1994 (2014).
Alicandro, G., Tavani, A. & La Vecchia, C. Coffee and cancer risk: a summary overview. Eur. J. Cancer Prev. http://dx.doi.org/10.1097/CEJ.0000000000000341 (2017).
Alisson-Silva, F., Kawanishi, K. & Varki, A. Human risk of diseases associated with red meat intake: analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Mol. Aspects Med. 51, 16–30 (2016).
Bao, Y. et al. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 369, 2001–2011 (2013). This work demonstrates a significant and intriguing inverse correlation between nut consumption and cancer-induced mortality.
Bonaccio, M. et al. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: prospective results from the Moli-sani study. Br. J. Nutr. 114, 804–811 (2015).
Siri-Tarino, P.W., Chiu, S., Bergeron, N. & Krauss, R.M. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu. Rev. Nutr. 35, 517–543 (2015).
Flint, T.R. et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 24, 672–684 (2016). This work elucidates how tumor-driven IL-6 production subverts the immunostimulatory effects induced by fasting and a ketogenic diet, thereby limiting anticancer immunity.
Sjöström, L. et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 10, 653–662 (2009).
Rossi, E.L. et al. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev. Res. (Phila.) 9, 339–348 (2016).
Lee, C. et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 4, 124ra27 (2012).
Di Biase, S. et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 (2016). This paper shows that a fasting-mimicking diet has immune-system-dependent anticancer activity, presumably through increasing the frequency of common CD8+ lymphocyte precursors in the bone marrow.
Cheng, C.W. et al. Fasting-mimicking diet promotes Ngn3-driven beta-cell regeneration to reverse diabetes. Cell 168, 775–788.e712 (2017).
Kreso, A. & Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 14, 275–291 (2014).
Madeo, F., Pietrocola, F., Eisenberg, T. & Kroemer, G. Caloric restriction mimetics: towards a molecular definition. Nat. Rev. Drug Discov. 13, 727–740 (2014).
Klement, R.J., Champ, C.E., Otto, C. & Kämmerer, U. Anti-tumor effects of ketogenic diets in mice: a meta-analysis. PLoS One 11, e0155050 (2016).
Woolf, E.C., Syed, N. & Scheck, A.C. Tumor metabolism, the ketogenic diet and β-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front. Mol. Neurosci. 9, 122 (2016).
Lussier, D.M. et al. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 16, 310 (2016).
Husain, Z., Huang, Y., Seth, P. & Sukhatme, V.P. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol. 191, 1486–1495 (2013).
Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330, 1029–1035 (1994).
Chen, A.C. et al. A Phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N. Engl. J. Med. 373, 1618–1626 (2015).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016). This pioneering paper reveals the antisenescence effects of NAD in a mammalian model of aging.
Acknowledgements
G.K. is supported by the Ligue contre le Cancer (Équipe Labelisée); Agence National de la Recherche (ANR)—Projets Blancs; ANR, under the framework of E-Rare-2, ERA-Net for Research on Rare Diseases; Association pour la Recherche sur le Cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); European Commission (ArtForce); European Research Council (ERC); LeDucq Foundation; LabEx Immuno-Oncology; RHU Torino Lumière, SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); SIRIC Cancer Research and Personalized Medicine (CARPEM); and Paris Alliance of Cancer Research Institutes (PACRI).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
L.Z. and G.K. are founders of the biotechnology company EverImmune.
Rights and permissions
About this article
Cite this article
Zitvogel, L., Pietrocola, F. & Kroemer, G. Nutrition, inflammation and cancer. Nat Immunol 18, 843–850 (2017). https://doi.org/10.1038/ni.3754
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3754
This article is cited by
-
ACSS2 controls PPARγ activity homeostasis to potentiate adipose-tissue plasticity
Cell Death & Differentiation (2024)
-
Clinical significance of preoperative CALLY index for prognostication in patients with esophageal squamous cell carcinoma undergoing surgery
Scientific Reports (2024)
-
Prognostic significance of pretreatment albumin–bilirubin (ALBI) grade and platelet–albumin–bilirubin (PALBI) grade in patients with small cell lung cancer
Scientific Reports (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Patient vulnerability is associated with poor prognosis following upfront hepatectomy for colorectal liver metastasis
International Journal of Clinical Oncology (2024)