Abstract
The FcμR receptor for the crystallizable fragment (Fc) of immunoglobulin M (IgM) can function as a cell-surface receptor for secreted IgM on a variety of cell types. We found here that FcμR was also expressed in the trans-Golgi network of developing B cells, where it constrained transport of the IgM-isotype BCR (IgM-BCR) but not of the IgD-isotype BCR (IgD-BCR). In the absence of FcμR, the surface expression of IgM-BCR was increased, which resulted in enhanced tonic BCR signaling. B-cell-specific deficiency in FcμR enhanced the spontaneous differentiation of B-1 cells, which resulted in increased serum concentrations of natural IgM and dysregulated homeostasis of B-2 cells; this caused the spontaneous formation of germinal centers, increased titers of serum autoantibodies and excessive accumulation of B cells. Thus, FcμR serves as a critical regulator of B cell biology by constraining the transport and cell-surface expression of IgM-BCR.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nguyen, X.H. et al. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood 118, 598–608 (2011).
Nguyen, X.H. et al. Antiapoptotic function of Toso (Faim3) in death receptor signaling. Blood 119, 1790–1791 (2012).
Hitoshi, Y. et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity 8, 461–471 (1998).
Kubagawa, H. et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J. Exp. Med. 206, 2779–2793 (2009).
Ouchida, R., Mori, H., Ohno, H. & Wang, J.Y. FcμR (Toso/Faim3) is not an inhibitor of Fas-mediated cell death in mouse T and B cells. Blood 121, 2368–2370 (2013).
Ouchida, R. et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc. Natl. Acad. Sci. USA 109, E2699–E2706 (2012).
Honjo, K. et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcμR). Proc. Natl. Acad. Sci. USA 109, 15882–15887 (2012).
Choi, S.C. et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J. Immunol. 190, 987–996 (2013).
Lang, K.S. et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc. Natl. Acad. Sci. USA 110, 2593–2598 (2013).
Brenner, D. et al. Toso controls encephalitogenic immune responses by dendritic cells and regulatory T cells. Proc. Natl. Acad. Sci. USA 111, 1060–1065 (2014).
Lang, P.A. et al. Toso regulates differentiation and activation of inflammatory dendritic cells during persistence-prone virus infection. Cell Death Differ. 22, 164–173 (2015).
Ouchida, R. et al. FcμR interacts and cooperates with the B cell receptor To promote B cell survival. J. Immunol. 194, 3096–3101 (2015).
Baeuerle, P.A. & Huttner, W.B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J. Cell Biol. 105, 2655–2664 (1987).
Lam, K.P., Kühn, R. & Rajewsky, K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90, 1073–1083 (1997).
Niiro, H. & Clark, E.A. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2, 945–956 (2002).
Vire, B., David, A. & Wiestner, A. TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J. Immunol. 187, 4040–4050 (2011).
Li, F.J. et al. Enhanced levels of both the membrane-bound and soluble forms of IgM Fc receptor (FcμR) in patients with chronic lymphocytic leukemia. Blood 118, 4902–4909 (2011).
Shima, H. et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int. Immunol. 22, 149–156 (2010).
Thorens, B., Schulz, M.F. & Vassalli, P. Bone marrow pre-B lymphocytes synthesize immunoglobulin mu chains of membrane type with different properties and intracellular pathways. EMBO J. 4, 361–368 (1985).
Nguyen, T.T., Elsner, R.A. & Baumgarth, N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J. Immunol. 194, 1489–1502 (2015).
Schermelleh, L., Heintzmann, R. & Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 190, 165–175 (2010).
Boes, M. et al. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160, 4776–4787 (1998).
Sieckmann, D.G., Stall, A.M. & Subbarao, B. A mouse monoclonal antibody specific for an allotypic determinant of the Igha allele of murine IgM: genetic and functional analysis of Igh-6a epitopes using anti-IgM monoclonal antibodies. Hybridoma 10, 121–135 (1991).
Choi, Y.S. & Baumgarth, N. Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 205, 3053–3064 (2008).
Lalor, P.A., Herzenberg, L.A., Adams, S. & Stall, A.M. Feedback regulation of murine Ly-1 B cell development. Eur. J. Immunol. 19, 507–513 (1989).
Andersson, J., Lafleur, L. & Melchers . IgM in bone marrow-derived lymphocytes. Synthesis, surface deposition, turnover and carbohydrate composition in unstimulated mouse B cells. Eur. J. Immunol. 4, 170–180 (1974).
Kläsener, K., Maity, P.C., Hobeika, E., Yang, J. & Reth, M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. eLife 3, e02069 (2014).
Yang, J. & Reth, M. Drosophila S2 Schneider cells: a useful tool for rebuilding and redesigning approaches in synthetic biology. Methods Mol. Biol. 813, 331–341 (2012).
Srinivasan, L. et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139, 573–586 (2009).
Werner, M., Hobeika, E. & Jumaa, H. Role of PI3K in the generation and survival of B cells. Immunol. Rev. 237, 55–71 (2010).
Coqueret, O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 13, 65–70 (2003).
Gogada, R. et al. Bim, a proapoptotic protein, up-regulated via transcription factor E2F1-dependent mechanism, functions as a prosurvival molecule in cancer. J. Biol. Chem. 288, 368–381 (2013).
Liu, P., Cheng, H., Roberts, T.M. & Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8, 627–644 (2009).
Hayakawa, K. et al. Positive selection of natural autoreactive B cells. Science 285, 113–116 (1999).
Berland, R. & Wortis, H.H. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20, 253–300 (2002).
Baumgarth, N. et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192, 271–280 (2000).
Ochsenbein, A.F. et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286, 2156–2159 (1999).
Pillai, S. & Cariappa, A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 9, 767–777 (2009).
Boes, M. et al. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl. Acad. Sci. USA 97, 1184–1189 (2000).
Jackson, S.W. et al. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J. Exp. Med. 213, 733–750 (2016).
Rahman, M.J. et al. Despite Increased Type 1 IFN, Autoimmune Nonobese Diabetic Mice Display Impaired Dendritic Cell Response to CpG and Decreased Nuclear Localization of IFN-Activated STAT1. J. Immunol. 196, 2031–2040 (2016).
Übelhart, R. et al. Responsiveness of B cells is regulated by the hinge region of IgD. Nat. Immunol. 16, 534–543 (2015).
Fuchs, E.J. & Matzinger, P. B cells turn off virgin but not memory T cells. Science 258, 1156–1159 (1992).
Baumgarth, N. et al. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA 96, 2250–2255 (1999).
Stall, A.M., Wells, S.M. & Lam, K.P. B-1 cells: unique origins and functions. Semin. Immunol. 8, 45–59 (1996).
Choi, Y.S., Dieter, J.A., Rothaeusler, K., Luo, Z. & Baumgarth, N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur. J. Immunol. 42, 120–129 (2012).
Doucett, V.P. et al. Enumeration and characterization of virus-specific B cells by multicolor flow cytometry. J. Immunol. Methods 303, 40–52 (2005).
Snapp, E.L., Altan, N. & Lippincott-Schwartz, J. Measuring protein mobility by photobleaching GFP chimeras in living cells. Current Protocols in Cell Biology (eds. Bonifacino, J.S. et al.) Ch. 21, Unit 21 (John Wiley and Sons, 2003).
Feder, T.J., Brust-Mascher, I., Slattery, J.P., Baird, B. & Webb, W.W. Constrained diffusion or immobile fraction on cell surfaces: a new interpretation. Biophys. J. 70, 2767–2773 (1996).
Lippincott-Schwartz, J. et al. Monitoring the dynamics and mobility of membrane proteins tagged with green fluorescent protein. Methods Cell Biol. 58, 261–281 (1999).
Reimold, A.M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Acknowledgements
We thank A. Spinner for help with flow cytometry; A. Treister for FlowJo software; R. Pohlmeyer for sharing flow cytometry data; J. Yang (University of Freiburg) for the GFP-IgD plasmid; M. Cavallari for help with image analysis; H. Kubagawa (Deutsches Rheuma Forschungszentrum) for BM from mice with global Fcmr deficiency; and the UC Davis Mouse Biology Program for generating Fcmrflx/flxCd19-Cre mice. Supported by the US National Institutes of Health (AI51354, AI85568 and U19AI109962 to N.B.), the UC Davis Graduate Group in Immunology, a Vietnamese Education Fellowship (T.T.T.N.), a UC Davis Chancellor's Fellowship (N.B.), the Excellence Initiative of the German Federal and State Governments (EXC 294), the European Research Council (322972) and the DFG (TRR130 and project 111026 of the German Cancer Aid to M.R.).
Author information
Authors and Affiliations
Contributions
T.T.T.N. and N.B. designed experiments, analyzed data and wrote the manuscripts; K.K., C.L.B. and M.R. provided help with experimental design; T.T.T.N., K.K., C.Z. and P.A.C. performed experiments; I.B.-M. and C.R. helped with STED and confocal microscopy and image analysis; D.M.I. performed pathological evaluation of Fcmrflx/flxCd19-Cre mice; and all authors provided edits to the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 FcμR is expressed by various cell subsets, including peripheral B cells.
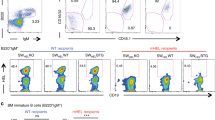
(a) Overlay histograms comparing surface FcμR expression in B cells, CD4 T cells, CD8 T cells, Gr-1+ (Granulocytes); CD11b+ and F4/80+ (Macrophages); CD11c+ (Dendritic Cells) from Fcmr flx/flxCd19-Cre and controls. (b) 5% contour plots with outliers of representative spleen samples from wild-type mice analyzed by flow cytometry after gating on live non-doublets. Gating shows FcμR+ of indicated cell subsets. Numbers in black indicate frequencies of FcμR+ cells among total live cells, and those in blue indicate frequencies of FcμR+ cells among CD19+, CD4+, CD8+, Gr-1+, CD11b+, and CD11c+ cells, respectively. Small contour plots are FMOs. (c) Overlay histograms show surface FcμR expression in different B cell subsets in spleen, peritoneal cavities (perc), and inguinal lymph nodes (pLN): spleen marginal zone (MZ), spleen follicular B (FO), spleen B-1, peritoneal cavity (perc) B-1, perc B-2, and peripheral lymph node (pLN) FO B cells. (d) Overlay histograms comparing surface FcμR expression in B cell precursors, including late pre B cells, immature B cells, and mature B cells.
Supplementary Figure 2 FcμR does not control BM B cell output.
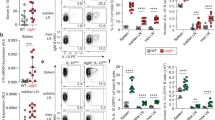
(a) Shown are frequencies (left) and numbers (right) of B cell precursors in Fcmr flx/flxCd19-Cre and control BMs (n=4 mice/group). (b,c) Fcmr flx/flxCd19-Cre and control mice (n = 3-4 mice/group) were sublethally irradiated (350rd) and peripheral B cell pools were analyzed 12 days after irradiation. Shown are the frequencies and numbers ± SD of (b) transitional (CD93+) and (c) total (CD19+) B cells in BM and spleen as assessed by multicolor flow cytometry. Data in (a) are representative of at least two independent experiments (mean ± SD in a-c). Data did not show significant differences by unpaired two-tailed Student’s t-test (p > 0.05).
Supplementary Figure 3 B cells rapidly lose surface-bound sIgM.
(a) Shown are histogram plots of splenic B cells from control and Fcmr flx/flxCd19-Cre mice after exposure to biotinylated sIgM (0h) and 0.5h after culture at 37oC. Binding was revealed by staining with streptavidin-Qdot605. (b,c) Mean fluorescent intensities (MFI) ± SD of sIgM binding to (b) B cells and (c) T cells in control and Fcmr flx/flxCd19-Cre mice at indicated times after culture as in (a). (d) To reveal sIgM binding to B cells in vivo, splenic B cells unable to secrete IgM (sIgM–/–) were isolated from mixed bone marrow sIgM–/– (IgMa) x wild type (IgMb) chimeras by autoMACS (n=3 mice). Graphs show MFI ± SD of sIgM binding (IgMb) to IgMa (sIgM–/–) B cells ex vivo and at indicated times during culture. Staining is compared to non-staining control (FMO “fluorescence minus one”) and staining for IgMb on B cells from sIgM–/– (IgMa) mice. n.s. not significantly different, *p<0.05, **p<0.005, ***p<0.0005 by unpaired t-test (a-b) and paired t-test (c).
Supplementary Figure 4 Monoclonal antibodies to IgMa (DS.1) and IgMb (AF6-78.25) are highly specific and bind only to their antigenic target.
(a) STED microscopy of IgHa and IgHb B cells stained for IgMa (red) and IgMb (green). (b) Binding of IgM using serum from wild type IgHa (red) and IgHb (green) wild type mice on ELISA plates coated and revealed with anti-IgMa (left) and IgMb (right). Graph showing mean O.D. per group (n=3-4 mice/group). (c) Overlay flow cytometry histograms showing IgMa (red) and IgMb (green) staining of B cells from congenic IgHa (left) and IgHb (middle) C57BL/6 mice, respectively. Graphs (right and far right) summarizing mean fluorescence intensities (MFI) ± SD of IgMa and IgMb staining for IgHa and IgHb B cells, respectively (n=3 mice/group). Data are compared to “fluorescence minus one” control stains. n.s. not significantly different by unpaired two-tailed Student’s t-test
Supplementary Figure 5 FcμR interacts with mIgM (BCR) in the TGN at the immature B cell stage but rarely in mature FO B cells.
Confocal microscopy of (a) spleen CD19+CD23+ follicular B cells from F1 mice generated by crossing secreted IgM-deficient sIgM–/– (IgHa) with wild type C57BL/6 (IgHb) mice. B cells from sIgM–/– mice were purified based on expression of IgDa and immunostained for secreted IgMb (green), mIgMa (red) and trans-Golgi stain TGN-38 (blue). Far right shows overlap of all three stains, identifying rare co-localization of mIgM but not sIgM with TGN-38. (b) CD19+ B220+ CD43– IgD– late-pre B/immature B cells were purified from C57BL/6 mice and stained for FcμR (green), IgM (red) and TGN-38. Far right shows overlap of all three stains. Note the strong co-localization of IgM with the FcμR in the trans-Golgi network. Scale bar 2μm. (c) Quantification of colocalization coefficients (n=40 cells), presented in a range from 0 (no co-localization) to 1 (total co-localization) (Manders et al., 1993). P, Pearson’s coefficient (R total value of co-localization); t M(1) and t M(2), extend of overlap of signal 1 (channel 1 488nm) with signal 2 (channel 2 PLA 545nm). Each symbol represents an individual cell, horizontal lines indicate the mean ± SD.
Supplementary Figure 6 Fcmr–/– B cells from BM chimeras with knockout of Fcmr show enhanced phosphorylated Akt and Btk.
Fcmr–/– and wild-type bone marrow chimeras were generated by i.v injecting Fcmr–/– (kindly provided by Dr. Hiromi Kubagawa) or wild type bone marrow cells into lethally irradiated C57BL/6 mice. Chimeras were allowed to reconstitute for at least 7 weeks before analysis. (a) Ex vivo pAkt and (b) pBtk expressions in total spleen B cells, follicular (FO) B (B-2) and B-1 cells from Fcmr–/– and control chimeras (n=4 mice/group). *p < 0.05, **p < 0.005, as assessed by comparing mean fluorescence intensities of stained cells (MFI) after subtraction of background MFI (mean ± SD in a-b), using unpaired two-tailed Student’s t-test.
Supplementary Figure 7 The presence of Cd19-Cre does not affect the number of B-1 cells or the PI3K pathway.
(a,b) Box-and-whisker-plots (min, max, median, quartiles) showing (a) ex vivo PI3K expressions and (b) ex vivo phostpho-Akt expressions in total spleen B cells from Fcmr wt/flxCd19-Cre– (Control), Fcmr wt/flxCd19-Cre+(Control), Fcmr flx/flxCd19-Cre– (Control) and Fcmr flx/flxCd19-Cre+ mice (n=4-5 mice/group). (c,d) Box-and-whisker-plots (min, max, median, quartiles) show numbers of B-1 cells, including B-1a and B-1b cells in (c) spleens and (d) peritoneal cavity. *p<0.05, **p<0.005, ***p<0.0005 by unpaired two-tailed Student’s t-test.
Supplementary Figure 8 Increased IgM production in chimeras reconstituted with Fcmr–/– B-1 cells and wild-type BM cells.
For lethally-irradiated B-1 chimeras, 2-months old μMT mice were lethally irradiated one day before transfer of 5 x 106 peritoneal lavage cells from Fcmr flx/flxCd19-Cre or control mice (IgHb) as a source of B-1 cells and BM cells from wild-type (IgHa) mice as a source of B-2 cells. Chimeras were rested for at least 7 weeks before analysis (n=4 mice/group). (a) Graphs summarize mean frequencies ± SD of IgMb+ secreting cells derived from B-1 cells and IgMa+ secreting cells derived form BM B-2 cells in spleens, and BMs as assessed by ELISPOT analysis. (b) Mean IgMb, IgMa, and IgM concentrations ± SD in sera of the chimeras, measured by ELISA. *p<0.05, **p<0.005, ***p<0.0005 by unpaired two-tailed Student’s t-test.
Supplementary Figure 9 Increases in B-1-cell-derived plasma cells in the absence of FcμR.
(a) Mean frequencies ± SD of IgM+ B-1 cell-derived plasma cells (CD19lowIgMbCD138+) in neonatal chimeras generated with Fcmr–/– or control B-1 cells as measured by multicolor flow cytometry (n=4 mice/group). Neonatal chimeras were created as outlined in Fig. 6d-g. (b) Mean frequencies ± SD of B-1 cell-derived IgM+ plasma cells (CD19lowIgMbCD138+) in BM chimeras generated with Fcmr–/– or control B-1 cells. BM irradiation chimeras were created as outlined in Supplementary Fig. 2 (n=4 mice/group). *p<0.05, **p<0.005, ***p<0.0005 by unpaired two-tailed Student’s t-test.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1379 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T., Kläsener, K., Zürn, C. et al. The IgM receptor FcμR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol 18, 321–333 (2017). https://doi.org/10.1038/ni.3677
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3677
This article is cited by
-
Secreted IgM modulates IL-10 expression in B cells
Nature Communications (2024)
-
Natural autoimmunity in oligoarticular juvenile idiopathic arthritis
Pediatric Rheumatology (2023)
-
Immunoglobulin M perception by FcμR
Nature (2023)
-
Multi-faceted immunoglobulin M meets its elusive receptor
Nature Structural & Molecular Biology (2023)
-
Structural basis for Fc receptor recognition of immunoglobulin M
Nature Structural & Molecular Biology (2023)