Abstract
Environmental challenges to epithelial cells trigger gene expression changes that elicit context-appropriate immune responses. We found that the chromatin remodeler Mi-2β controls epidermal homeostasis by regulating the genes involved in keratinocyte and immune-cell activation to maintain an inactive state. Mi-2β depletion resulted in rapid deployment of both a pro-inflammatory and an immunosuppressive response in the skin. A key target of Mi-2β in keratinocytes is the pro-inflammatory cytokine thymic stromal lymphopoietin (TSLP). Loss of TSLP receptor (TSLPR) signaling specifically in regulatory T (Treg) cells prevented their activation and permitted rapid progression from a skin pro-inflammatory response to a lethal systemic condition. Thus, in addition to their well-characterized role in pro-inflammatory responses, keratinocytes also directly support immune-suppressive responses that are critical for re-establishing organismal homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Pasparakis, M., Haase, I. & Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 14, 289–301 (2014).
Kupper, T.S. & Fuhlbrigge, R.C. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4, 211–222 (2004).
Rangwala, S. & Tsai, K.Y. Roles of the immune system in skin cancer. Br. J. Dermatol. 165, 953–965 (2011).
Liu, Y.J. et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25, 193–219 (2007).
Wilson, S.R. et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295 (2013).
Volpe, E. et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J. Allergy Clin. Immunol. 134, 373–381 (2014).
Guttman-Yassky, E. et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 181, 7420–7427 (2008).
Demehri, S. et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 22, 494–505 (2012).
Di Piazza, M., Nowell, C.S., Koch, U., Durham, A.D. & Radtke, F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 22, 479–493 (2012).
Kim, J. et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10, 345–355 (1999).
Yoshida, T. et al. The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation. Genes Dev. 22, 1174–1189 (2008).
Williams, C.J. et al. The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity 20, 719–733 (2004).
Gómez-Del Arco, P. et al. The chromatin remodeling complex Chd4/NuRD controls striated muscle identity and metabolic homeostasis. Cell Metab. 23, 881–892 (2016).
Kashiwagi, M., Morgan, B.A. & Georgopoulos, K. The chromatin remodeler Mi-2beta is required for establishment of the basal epidermis and normal differentiation of its progeny. Development 134, 1571–1582 (2007).
Werner, S. & Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835–870 (2003).
Weaver, C.T. & Hatton, R.D. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 9, 883–889 (2009).
Mohammed, J. et al. TGFβ1 overexpression by keratinocytes alters skin dendritic cell homeostasis and enhances contact hypersensitivity. J. Invest. Dermatol. 133, 135–143 (2013).
Yasmin, N. et al. Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J. Exp. Med. 210, 2597–2610 (2013).
Strid, J., Sobolev, O., Zafirova, B., Polic, B. & Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 334, 1293–1297 (2011).
Willcox, C.R. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13, 872–879 (2012).
Witherden, D.A. et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity 37, 314–325 (2012).
Stumptner-Cuvelette, P. & Benaroch, P. Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta 1542, 1–13 (2002).
Deane, J.A. et al. Endogenous regulatory T cells adhere in inflamed dermal vessels via ICAM-1: association with regulation of effector leukocyte adhesion. J. Immunol. 188, 2179–2188 (2012).
Kolls, J.K., McCray, P.B. Jr. & Chan, Y.R. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol. 8, 829–835 (2008).
Li, M. et al. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc. Natl. Acad. Sci. USA 102, 14795–14800 (2005).
Li, M. et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 103, 11736–11741 (2006).
Li, J. et al. Counterregulation between thymic stromal lymphopoietin- and IL-23-driven immune axes shapes skin inflammation in mice with epidermal barrier defects. J. Allergy Clin. Immunol. 138, 150–161 e113 (2016).
He, R. et al. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc. Natl. Acad. Sci. USA 105, 11875–11880 (2008).
Demehri, S. et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 6, e123 (2008).
Dumortier, A. et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One 5, e9258 (2010).
Lee, H.C. & Ziegler, S.F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc. Natl. Acad. Sci. USA 104, 914–919 (2007).
Fontenot, J.D., Gavin, M.A. & Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Vignali, D.A., Collison, L.W. & Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008).
Campbell, D.J. & Koch, M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11, 119–130 (2011).
Feuerer, M., Hill, J.A., Mathis, D. & Benoist, C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 10, 689–695 (2009).
Chen, X., Bäumel, M., Männel, D.N., Howard, O.M. & Oppenheim, J.J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol. 179, 154–161 (2007).
Setiady, Y.Y., Coccia, J.A. & Park, P.U. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur. J. Immunol. 40, 780–786 (2010).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Quintana, F.J. et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 13, 770–777 (2012).
Cretney, E., Kallies, A. & Nutt, S.L. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 34, 74–80 (2013).
Tomura, M. et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Invest. 120, 883–893 (2010).
Dudda, J.C., Perdue, N., Bachtanian, E. & Campbell, D.J. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J. Exp. Med. 205, 1559–1565 (2008).
Shevach, E.M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009).
Joller, N. et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 40, 569–581 (2014).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002).
Chinen, T. et al. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 17, 1322–1333 (2016).
Rochman, Y. et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl. Acad. Sci. USA 107, 19455–19460 (2010).
Bell, B.D. et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat. Immunol. 14, 364–371 (2013).
Isaksen, D.E. et al. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J. Immunol. 163, 5971–5977 (1999).
Carpino, N. et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell. Biol. 24, 2584–2592 (2004).
Han, H., Thelen, T.D., Comeau, M.R. & Ziegler, S.F. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J. Clin. Invest. 124, 5442–5452 (2014).
Metzger, D., Li, M. & Chambon, P. Targeted somatic mutagenesis in the mouse epidermis. Methods Mol. Biol. 289, 329–340 (2005).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Huang, W., Sherman, B.T. & Lempicki, R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Zhang, J. et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat. Immunol. 13, 86–94 (2011).
Acknowledgements
We thank P. Chambon (Institute of Genetics and Cellular and Molecular Biology) and V.K. Kuchroo (Brigham and Women's Hospital) for the Krt14-Cre-ERT2 mice and Foxp3-IRES-EGFP mice, respectively. We thank M.E. Bigby for consultation on lymphocyte isolation from the skin and L.M. Francisco and A.H. Sharpe for consultation on Treg cell and DC analysis. We thank E. Wu and B. Czyzewski for mouse husbandry, A. Cho, M. Ahl, J.E. King and J. Brandollni for bone marrow transplantation, and T. Minegishi for R platform support. We also thank J.M. Park, H. Cantor, H.J. Kim, F. Gounari and K. Khashayarsha for critical comments on the manuscript. This research was supported by NIH R21 AR055813, RO1 AR064390 and R01AR069132 to K.G., NIH RO1 AI068731 and PO1 HL098067 to S.F.Z., and NIH R01 AR055256 to B.A.M. K.G. is an MGH scholar supported by J. de Gunzburg. J.H. was supported by a Shiseido grant. High-throughput RNA sequencing was performed at the Bauer Center for Genomic research Harvard University, Cambridge.
Author information
Authors and Affiliations
Contributions
M.K. designed and performed experiments and analyzed experimental data. J.H. designed and executed cytokine studies on primary cultured keratinocytes and advised on DC and DETC studies. J.-F.L. provided experimental materials and advised on immunological studies. J.B. advised on keratinocyte transcriptional studies. S.F.Z. provided experimental materials and guidance throughout the project. K.G and B.A.M supervised research and analyzed data. M.K., K.G. and B.A.M wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Pro-inflammatory responses in the Mi-2β deficient skin
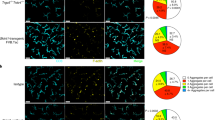
(a) Clinical pictures of mice with Mi-2β depleted epidermis showing flaky skin in the shaved dorsal area and around the eyes. (b) Flow cytometric analysis of single cell suspension from wild-type and Mi2Δ ear skin stained for CD45, CD4 and CD3ɛ. The clinical pictures shown in (a) was seen with more than ten Mi2D mice. Data shown in (b) were representative of four independent experiments performed on a total of WT N=6 and Mi2Δ N=6 mice.
Supplementary Figure 2 The role of Mi-2β in the regulation of keratinocyte-specific gene networks
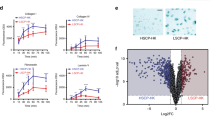
(a) Flow cytometric analysis of single cell suspension stained for ITGA6, CD45 and CD34 in wild-type and Mi2Δ epidermis. mRNAs were isolated from FACS-sorted ITGA6+CD45–CD34– basal keratinocytes and subjected to RNA-sequencing. (b) Expression of genes relevant to keratinocytes activation and migration are shown as normalized exon mapping reads (mean+s.e.m.). (c) A model is shown of Mi-2β-based negative regulation of gene networks in keratinocytes in steady state epidermis and how this effect is reversed upon Mi-2β depletion. Data shown in (a-b) were generated from two independent experimental groups with pooled samples from WT N=6 and Mi2Δ N=4 mice.
Supplementary Figure 3 Activation of Mi2Δ keratinocytes and DETCs is independent of TSLPR signaling
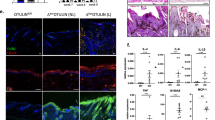
(a) Representative images of hematoxylin and eosin staining of dorsal skin from Mi2Δ, TMKO, RMKO and littermate controls were shown at day 7 and day 9 after induction of Mi-2β deletion in the skin. At day 9, skin hyperplasia was seen in Mi-2β mutant skin regardless of the presence of lymphocytes (Mi2Δ and RMKO), but was milder in RMKO mice. Scale bar, 50μm. WT and Mi2Δ sections shown for day 9 are also used in Fig. 1d. (b) DETCs (CD3+) and LCs (CD207+) in epidermal sheets from the ear were detected by immunofluorecence. The rounded morphology of DETCs, indicative of their activation, was seen in the Mi2Δ epidermis regardless of TSLPR signaling. Scale bar, 20μm. Data shown were representative of three independent experiments with more than five mice per group.
Supplementary Figure 4 Activation of Mi2Δ skin DCs is independent of TSLPR signaling
(a-b) Flow cytometric analysis of MHCclassIIhi dendritic populations in sDLNs from wild-type, TSLPRΔ, Mi2Δ, TMKO (a) and RagΔ and RMKO (b) mice. Three major populations were revealed by staining for CD207 (Langerin), EpCAM, and CD11c. The activation and maturation state of DCs was measured by expression of CD40, and CD86 in MHCclassIIhiEpCAMhiCD207+ LCs (red) and MHCclassIIhiEpCAMloCD207– DCs (blue). In Mi2Δ skin both LCs and other DCs were increased and showed an activated phenotype, regardless of the Rag or TSLPR mutations. Data shown were representative of three independent experiments with more than five mice per group.
Supplementary Figure 5 Activation of Mi2Δ skin Treg cells is dependent for TSLPR signaling
(a) Absolute cell numbers of CD4+ and CD8+ T cells in sDLNs are shown. *P < 0.05, **P < 0.01, ***P < 0.0001 (two-tailed unpaired t-test). (b) A full version of the Treg cell and Teff cell analysis shown in Fig. 4b is presented here. Expression of CD25, CD44, TNFR2, CTLA4, KLRG1, CD103 and the Ki67 antigen was analyzed in CD4+Foxp3+ Treg cells and CD44 and CD69 was analyzed in CD4+Foxp3- Teff cells from wild-type, TSLPRΔ, Mi2Δ, and TMKO sDLNs. Percent of cells in the gate or MFI for these markers are shown. (c) In vitro Treg cell immunosuppressive assay. CD4+CD62L+Foxp3-GFP- cells (Teff) were co-cultured with CD4+Foxp3-GFP+ cells (Treg) from Mi2Δ sDLN at indicated ratios (Teff: Treg) and stimulated with anti-CD3ɛ in the presence of irradiated-APCs for 3 days. The effect on Teff proliferation was assessed by the change in PI+ S phase cells. Treg cells from sDLNs or spleen of wild-type mice were used as a control. (d) CD4+Foxp3- Teff cells were stimulated in vitro and tested for intracellular expression of pro-inflammatory cytokines. CD4+Foxp3-GFP– T cells from wild-type, Mi2Δ and TMKO sDLN were stimulated with anti-CD3ɛ and anti-CD28 for 16 h. Cells were re-stimulated with PMA and ionomycin for 4 h and stained for intracellular IL-2, TNF and IFNγ. (–); without PMA or ionomycin, (+); with PMA and ionomycin. Data shown in (a) were generated from five independent experiments with a total of WT N=5, TSLPRΔ N=5, Mi2Δ N=10, and TMKO N=10 mice (mean+/– s.e.m). Data shown in (b) were representative of four independent experiments with WT N=5, TSLPRΔ N=5, Mi2Δ N=4, TMKO, N=4 mice, in (c-d) were representative of two experiments with pooled samples from WT N=14 and Mi2Δ N=5 mice.
Supplementary Figure 6 Direct induction of Treg cell activation by TSLPR signaling
(a-b) Analysis of CD4+Foxp3+CD25+ Treg cells in the skin and sDLNs of chimeric wild-type or Mi2D mice is shown. TSLPRΔ (donor) and wild-type (recipient) Treg cells were distinguished by Foxp3-GFP expression in the recipient population. (c) Ratios of TSLPRΔ to wild-type Treg cells in skin and sDLN are shown. *P < 0.01 (two-tailed unpaired t-test). (d) Disease development and staging for 4-OHT treated wild-type and Mi2Δ chimeras is shown. Data were generated from three independent experiments with WT N=5 and Mi2Δ N=5 chimeric mice.
Supplementary Figure 7 Treg cell immunosuppression is directly dependent on TSLPR signaling
Flow cytometric analysis of CD4+ T cells from skin and sDLNs, for Foxp3 and CD25 expression is shown in (a), and for Foxp3, CTLA4 and CD103 expression is shown in (b). Both a reduction in activation and number of TSLPRΔ Treg cells was seen. Data were representative of four independent experiments with Rag: TSLPR+ Treg N=5, Rag:TSLPRD Treg N=5, RMKO:TSLPR+ Treg N=4, and RMKO:TSLPRD Treg N=4 chimeric mice.
Supplementary Figure 8 IL-2R signaling is not required for Treg cell activation in Mi2Δ skin
(a) Schema of anti-CD25 treatment and induction of Mi-2β deletion with 4-OHT with time points of analysis. (b-c) Flow cytometric analysis of single cell suspension staining for CD3ɛ, CD4, CD25 and Foxp3 in WT and Mi2Δ skin with or without anti-CD25 treatment. Data shown are representative of three independent experiments, two at day 8 and one at day 14 after Mi-2β deletion with WT:PBS N=3, WT:anti-CD25 N=4, Mi2Δ:PBS N=2 and Mi2Δ:anti-CD25 N=4 mice.
Supplementary Figure 9 Direct control of regulatory T cells by keratinocytes
A model on how a pro-inflammatory signal overrides the repressive activity of the Mi-2β/NuRD complex in keratinocytes to induce TSLP expression and activate TSLPR signaling in skin-associated Treg cells. TSLPR signaling in Treg cells leads to the rapid induction of soluble and membrane bound immunosuppressive factors that are critical for an early Treg cell immunosuppressive function that prevents development of a systemic inflammatory response with dire for the organism consequences.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 2699 kb)
Supplementary Table
Supplementary Table 1 (XLSX 342 kb)
Supplementary Table
Supplementary Table 2 (XLSX 256 kb)
Supplementary Table
Supplementary Table 3 (XLSX 54 kb)
Rights and permissions
About this article
Cite this article
Kashiwagi, M., Hosoi, J., Lai, JF. et al. Direct control of regulatory T cells by keratinocytes. Nat Immunol 18, 334–343 (2017). https://doi.org/10.1038/ni.3661
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3661
This article is cited by
-
Mi-2β promotes immune evasion in melanoma by activating EZH2 methylation
Nature Communications (2024)
-
Epithelial cell-derived cytokine TSLP activates regulatory T cells by enhancing fatty acid uptake
Scientific Reports (2023)
-
Mining chicken ileal microbiota for immunomodulatory microorganisms
The ISME Journal (2023)
-
Advances in the modulation of ROS and transdermal administration for anti-psoriatic nanotherapies
Journal of Nanobiotechnology (2022)
-
Combined application of dinitrofluorobenzene and ovalbumin induced AD-like dermatitis with an increase in helper T-cell cytokines and a prolonged Th2 response
BMC Immunology (2022)