Abstract
The innate responsiveness of the immune system is important not only for quick responses to pathogens but also for the initiation and shaping of the subsequent adaptive immune response. Activation via the cytokine IL-18, a product of inflammasomes, gives rise to a rapid response that includes the production of self-reactive antibodies. As increased concentrations of this cytokine are found in inflammatory diseases, we investigated the origin of the B cell response and its regulation. We identified an accumulation of B cell–helper neutrophils in the spleen that interacted with innate-type invariant natural killer T cells (iNKT cells) to regulate B cell responses. We found that neutrophil-dependent expression of the death-receptor ligand FasL by iNKT cells was needed to restrict autoantibody production. Neutrophils can thus license iNKT cells to regulate potentially harmful autoreactive B cell responses during inflammasome-driven inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sedimbi, S.K., Hägglöf, T. & Karlsson, M.C. IL-18 in inflammatory and autoimmune disease. Cell. Mol. Life Sci. 70, 4795–4808 (2013).
Sims, J.E. & Smith, D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Boraschi, D. & Dinarello, C.A. IL-18 in autoimmunity: review. Eur. Cytokine Netw. 17, 224–252 (2006).
Tanaka, T. et al. Interleukin-18 is elevated in the sera from patients with atopic dermatitis and from atopic dermatitis model mice, NC/Nga. Int. Arch. Allergy Immunol. 125, 236–240 (2001).
Lind, S.M. et al. IL-18 skews the invariant NKT-cell population via autoreactive activation in atopic eczema. Eur. J. Immunol. 39, 2293–2301 (2009).
Enoksson, S.L. et al. The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc. Natl. Acad. Sci. USA 108, E1399–E1407 (2011).
Wermeling, F., Lind, S.M., Jordö, E.D., Cardell, S.L. & Karlsson, M.C. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J. Exp. Med. 207, 943–952 (2010).
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006).
Donadieu, J., Beaupain, B., Mahlaoui, N. & Bellanne-Chantelot, C. Epidemiology of congenital neutropenia. Hematol. Oncol. Clin. 27, 1–17 (2013).
Hostetter, S.J. Neutrophil function in small animals. Vet. Clin. North Am. Small Anim. Pract. 42, 157–171 (2012).
Jönsson, F., Mancardi, D.A., Albanesi, M. & Bruhns, P. Neutrophils in local and systemic antibody-dependent inflammatory and anaphylactic reactions. J. Leukoc. Biol. 94, 643–656 (2013).
Parsa, R. et al. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J. Exp. Med. 213, 1537–1553 (2016).
Puga, I. et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13, 170–180 (2011).
De Santo, C. et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 11, 1039–1046 (2010).
Wingender, G. et al. Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J. Immunol. 188, 3000–3008 (2012).
Bosch, X. Systemic lupus erythematosus and the neutrophil. N. Engl. J. Med. 365, 758–760 (2011).
Garcia-Romo, G.S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Mantovani, A., Cassatella, M.A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Scapini, P. et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 197, 297–302 (2003).
Figgett, W.A. et al. The TACI receptor regulates T-cell-independent marginal zone B cell responses through innate activation-induced cell death. Immunity 39, 573–583 (2013).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Germanov, E. et al. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J. Immunol. 181, 81–91 (2008).
King, I.L. et al. The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J. Immunol. 191, 572–582 (2013).
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Wu, L. & Van Kaer, L. Natural killer T cells and autoimmune disease. Curr. Mol. Med. 9, 4–14 (2009).
Green, M.R. et al. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IGG production. Arthritis Rheum. 56, 303–310 (2007).
Wilson, M.T. et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. USA 100, 10913–10918 (2003).
Kovalovsky, D. et al. The BTB–zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 9, 1055–1064 (2008).
Wei, G. et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311 (2011).
MacLennan, I.C. et al. Extrafollicular antibody responses. Immunol. Rev. 194, 8–18 (2003).
Luzina, I.G. et al. Spontaneous formation of germinal centers in autoimmune mice. J. Leukoc. Biol. 70, 578–584 (2001).
William, J., Euler, C., Christensen, S. & Shlomchik, M.J. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297, 2066–2070 (2002).
Barral, P., Sánchez-Niño, M.D., van Rooijen, N., Cerundolo, V. & Batista, F.D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 31, 2378–2390 (2012).
Chang, P.P. et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43 (2011).
Coquery, C.M. et al. Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus-prone mice. PLoS One 9, e102284 (2014).
Lee, Y.J., Holzapfel, K.L., Zhu, J., Jameson, S.C. & Hogquist, K.A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154 (2013).
Lynch, L. et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol. 16, 85–95 (2015).
Engel, I. et al. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat. Immunol. 17, 728–739 (2016).
Kain, L. et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity 41, 543–554 (2014).
Birkholz, A.M., Howell, A.R. & Kronenberg, M. The alpha and omega of galactosylceramides in T cell immune function. J. Biol. Chem. 290, 20746 (2015).
Seifert, M. et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc. Natl. Acad. Sci. USA 112, E546–E555 (2015).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Chen, Y. et al. A regulatory role for macrophage class A scavenger receptors in TLR4-mediated LPS responses. Eur. J. Immunol. 40, 1451–1460 (2010).
Acknowledgements
We thank M. Tighe, A. Duhlin, E. Lind and P. Lanthier for technical assistance; M. Winerdal for illustrations; the US National Institutes of Health tetramer core facility for CD1d tetramers; M. Johansson and P. Höglund (Karolinska Institutet) for iNKT cell–deficient C57BL/6 Cd1d1−/− mice and C57BL/6 Traj18−/− mice backcrossed for more than ten generations; and S. Smiley (Trudeau Institute) for Pad4−/− mice on a BALB/c background. Supported by the Swedish Research Council, The Center for Allergy Research Karolinska Institutet, The Swedish Medical Society, King Gustaf V's 80-year Foundation, the Magnus Bergvall Foundation, the Karolinska Institutet (M.C.I.K.), the Erik and Edith Fernström foundation, the Swedish Society for Medical Research, the Centre for Allergy Research, the Ellen, Walter and Lennart Hesselman foundation (T.H.), O.E. and Edla Johansson's Foundation, Tore Nilsson's foundation, Karolinska Institutet's foundations (S.K.S.), the US National Institutes of Health (AI104788-01A1 to E.A.L.), the Trudeau Institute and the University of Texas Health Science Center at San Antonio (E.A.L.).
Author information
Authors and Affiliations
Contributions
T.H., S.K.S., R.P., E.A.L. and M.C.I.K. designed experiments; T.H., S.K.S., J.L.Y., R.P. and B.H.S. performed experiments; T.H., S.K.S., E.A.L., R.P., B.H.S. and M.C.I.K. analyzed and interpreted the data; T.H., E.A.L. and M.C.I.K. wrote the manuscript; and T.H., S.K.S., J.L.Y., R.P., B.H.S., R.A.H., E.A.L. and M.C.I.K. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
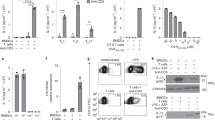
Supplementary Figure 1 Leukocyte expression of IL-18R, BAFF and Fas, plus evaluation of Pad4–/– mice.
(a) Flow cytometry detection of IL-18R on mouse naïve splenic neutrophils and follicular B cells. (b) Quantitative RT-PCR analysis of mRNA encoding BAFF (Tnfsf13b) in sorted splenic macrophages freshly isolated from vehicle or IL-18 injected mice at day 12. Results are normalized to mRNA encoding Hprt1 to determine mean fold change, and are presented as relative expression (RE) compared with splenic macrophages after vehicle treatment. (c) Flow cytometry of neutrophils cultured 16 h in vitro with medium (left panels) or 20 ng/ml IL-18 (right panels). Neutrophils were labeled with isotype control (top panels) or anti-BAFF antibody (clone 121808; bottom panels). Boxes indicate BAFF+ neutrophils. (d) Flow cytometry of frequency of CD11b+ Ly6G—macrophages (MØ) expressing surface BAFF following 16 h in vitro culture in the presence of 20 ng/ml IL-18 or medium. (e) Flow cytometry determination of frequencies of splenic B220+ B cells expressing Fas following co-culture with or without neutrophils and 20 ng/ml IL-18 or medium for 6 h, as noted. Anti-BAFF antibody or BAFF–R–Ig was added to the cultures to block BAFF. (f) Confocal immunofluorescence of neutrophil elastase in the spleens of wild-type (C57BL/6) mice treated with vehicle (left) or IL-18 (right) at day 12. (n = 3 per group). Scale bar, 100 μm. (g) ELISA measure of serum titers of total IgE and (h) anti-DNA IgM, IgG, and IgE antibodies (day 12) in homozygous Pad4–/– mice and heterozygous littermate controls on day 12 after injections with IL-18 or vehicle. *P < 0.05 [two-tailed unpaired Student’s t-test (b; n = 4 per group, 3 technical replicates), (d, n= 6 per group, 3 technical replicates), 2way ANOVA with Sidak correction (e, n= 5 per group, two technical replicates) or (g, h; n = 5 per group). Data are representative of one (f), two (e), three (c) experiments or pooled from two (d) or five experiments (g, h). Box height indicates mean + s.e.m.
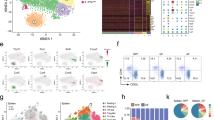
Supplementary Figure 2 Mouse leukocyte CD1d expression and gating strategy for CD1d–/– mixed-BM chimeras.
(a) Flow cytometry plots of Ly6G+ CD11b+ neutrophils (upper left panel) and B220+ CD21+ CD23lo MZ B cells (upper right panel). CD1d expression of gated populations is displayed in corresponding lower panels. Black line represents WT (C57BL/6) mice, filled histogram represents CD1d–/– mice. (b) CD1d expression by CD8+ T cells (left panel) and CD11b+ Ly6G- macrophages (right panel) following 16 h in vitro stimulation with IL-18 (blue line) or vehicle (filled histogram). (c) Confocal immunofluorescence of day 12 IL-18 treated WT (C57BL/6) spleen stained for B220 (white), Ly6G (red), CD1d (blue), and CXCR6-GFP (green) reported. Boxed inset is magnified and displayed in right panel, the arrow indicates a neutrophil and iNKT cell in close proximity. Scale bar, 100 μm. (d) Flow cytometry gating strategy to identify CD1d–/– or WT neutrophils using congenic markers. Numbers in upper left panel indicate frequency of congenic populations. Gate in upper right panel identifies neutrophils. Expression of CD1d for WT (CD45.1+; lower left panel) and CD1d–/– (CD45.1-, lower right panel) neutrophils. Neutrophils from vehicle-injected controls in filled histograms and from IL-18-injected mice in colored histograms.
Supplementary Figure 3 iNKT cell and T cell phenotypes, plus leukocyte repopulation following neutrophil depletion during inflammation.
(a) Flow cytometry measure of percentage of CD69+ CD1d-tet+ iNKT cells (left panel) and MFI level on CD1d-tet+ iNKT cells (right panel) 4 and 12 hrs after IL-18 or vehicle administration to wild type (C57BL/6) mice in vivo. (b) Frequency of splenic γδT cells in vehicle or IL-18 injected mice at day 12 as assessed by flow cytometry. (c) Flow cytometry plots displaying representative neutrophil populations in mouse spleen (top panels) or blood (bottom panels) on day 12 following treatments indicated. Depletion antibody (anti-Ly6G- clone 1A8) administered on days 0 and 4. (d) Summary of flow cytometry data shown in (c), displaying kinetics of neutrophil numbers in spleen (upper panel) and blood (lower panel) in mice depleted at days 0 and 4. (e) Flow cytometry determination of number of splenic iNKT cells following treatment as indicated (depletion as in c). (f) ELISA measure of serum BAFF in mice treated as indicated. Data expressed as % of BAFF detected in vehicle treated mice. (g) Cytometric bead array kinetic measure of serum concentrations of IFN–γ from IL-18 or vehicle treated wild-type (C57BL/6) mice in the presence or absence of neutrophil depleting antibody (1A8) given days 0 and 4. (h) Intracellular flow cytometry data displaying MFI values for T-bet expression by iNKT cells in vehicle or IL-18 injected mice on day 12. (i) Intracellular flow cytometry data displaying MFI values for CD4+ and CD8+ T cell expression of the nuclear transcription factor GATA3 on day 12 after injection with IL-18 or vehicle. (j) Flow cytometry measure of FasL expression for conventional CD1d-tet– T cells after IL-18 injection, normalized to levels in cells from vehicle treated mice. *P < 0.05 (two-tailed unpaired Student’s t-test [(a, n = 3 per group), (b, d; n = 4 per group), (e; n = 8 mice per group), (h; n = 5 mice per group), (i; n = 5 mice per group), (j; 14 mice per group)] or [1way ANOVA with Tukey correction (f; n = 8 per group), (g; n = 5 per group)]. Data are representative of one (a, g), two (h, i), or pooled from two (b, e, j), three (f), or four experiments (d). Box height or symbol center indicates group mean + s.e.m.
Supplementary Figure 4 Characterization of iNKT cell–specific Fasl–/– chimeras.
(a) Flow cytometry data showing congenic marker expression (CD45.1+ vs CD45.2+) of cells from mixed BM chimeras generated by transfer of Traj18–/– + wild-type or Traj18–/– + Fasl–/– BM into Traj18–/– recipients. (b) Flow cytometry data showing gating strategy for iNKT cells in spleens of mixed BM chimeras discussed in (a). Congenic markers are used to identify lymphocytes derived from Traj18–/– (CD45.1+; left panels) vs wild-type or Fasl–/– (CD45.2+; right panels) BM within the same mouse. (c) Flow cytometry histograms showing expression of FasL in iNKT cells (top) and CD8+ T cells (bottom), gated as in (b). Filled histograms indicate expression in Fasl–/– cells; red histograms indicate expression in wild-type cells. (d) Flow cytometry data showing proportions of iNKT cells in chimeric mice reconstituted with wild-type [iNKT(WT FasL)] or Fasl–/– iNKT cells [iNKT(ΔFasL)]. *P < 0.05 (two-tailed unpaired Student’s t-test [(d; n = 4 per group). Data represents three independent experiments (a-d). Box height indicates group mean + s.e.m.
Supplementary Figure 5 Activation-marker expression by mouse B cell subsets and leukocyte characterization after continuous neutrophil depletion during IL-18-induced inflammation.
(a) Flow cytometry data showing frequencies of B cell subsets in wild-type (C57BL/6) compared to CD1d1–/– mice following injections with vehicle or IL-18, day 12. (b) ELISA measures of serum amounts of total IgM (left panel) and IgG (right panel) in WT and CD1d1–/– mice injected with vehicle or IL-18, day 12. (c-e) Flow cytometric analysis of activation marker expression by indicated B cell subset in the spleens of wild-type (C57BL/6) or CD1d1–/– mice following treatment with vehicle or IL-18, day 12. (f) Representative flow cytometry plots of splenic neutrophils on day 12 following vehicle or IL-18 treatment in combination with doubled neutrophil depletion strategy (Depl) using 500 μg of 1A8 antibody 24 h before IL-18 and on days 3, 6, and 9 in wild-type (C57BL/6) mice. Numbers below outlined areas indicate percent Ly6G+ CD11b+ neutrophils. (g) Summary of flow cytometry assessment of frequency of splenic Ly6G+ CD11b+ neutrophils from (g). (h) Left panel: Flow cytometry assessment of frequency of splenic CD138+ B220lo PCs day 12 after vehicle or IL-18 treatment in combination with neutrophil depletion according to (f). Center panel: Flow cytometry assessment of frequency of splenic B220+ CD95+ GL7+ GC B cells day 12 after vehicle or IL-18 treatment in combination with neutrophil depletion according to (f). Right panel: ELISA measure of serum concentrations of anti-DNA IgG antibodies in mice day 12 after vehicle or IL-18 treatment in combination with neutrophil depletion according to (f). (i) Intracellular flow cytometry assessment of expression in iNKT cells of the nuclear transcription factor GATA3 day 12 after vehicle or IL-18 treatment in combination with neutrophil depletion according to (f). *P < 0.05 (two-tailed unpaired Student’s t-test; wild-type (C57BL/6) IL-18 vs CD1d1–/– IL18 groups) (a-e; n = 5 mice per group) or (1way ANOVA with Tukey correction) (g-i; n = 4 per group). Data pooled from two experiments (a-e) or from one experiment (f-i). Box height indicates group mean + s.e.m.
Supplementary Figure 6 Autoreactive B cells are regulated by neutrophil-licensed iNKT cells during inflammation.
Neutrophil-dependent FasL expression by iNKT cells is required to restrict autoantibody production. Neutrophils can thus license iNKT cells to regulate potentially harmful autoreactive B cell responses during inflammasome-driven inflammation.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1859 kb)
Supplementary Table 1
Antibody Summary (PDF 209 kb)
Rights and permissions
About this article
Cite this article
Hägglöf, T., Sedimbi, S., Yates, J. et al. Neutrophils license iNKT cells to regulate self-reactive mouse B cell responses. Nat Immunol 17, 1407–1414 (2016). https://doi.org/10.1038/ni.3583
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3583
This article is cited by
-
Host-derived lipids orchestrate pulmonary γδ T cell response to provide early protection against influenza virus infection
Nature Communications (2021)
-
The functional diversity of neutrophils and clustered polarization of immunity
Cellular & Molecular Immunology (2020)
-
Extracellular annexin-A1 promotes myeloid/granulocytic differentiation of hematopoietic stem/progenitor cells via the Ca2+/MAPK signalling transduction pathway
Cell Death Discovery (2019)
-
CD1: A Singed Cat of the Three Antigen Presentation Systems
Archivum Immunologiae et Therapiae Experimentalis (2017)