Abstract
Hematopoietic stem cells (HSCs) are dormant in the bone marrow and can be activated in response to diverse stresses to replenish all blood cell types. We identified the ubiquitin ligase Huwe1 as a crucial regulator of HSC function via its post-translational control of the oncoprotein N-myc (encoded by Mycn). We found Huwe1 to be essential for HSC self-renewal, quiescence and lymphoid-fate specification in mice. Through the use of a fluorescent fusion allele (MycnM), we observed that N-myc expression was restricted to the most immature, multipotent stem and progenitor populations. N-myc expression was upregulated in response to stress or following loss of Huwe1, which led to increased proliferation and stem-cell exhaustion. Mycn depletion reversed most of these phenotypes in vivo, which suggested that the attenuation of N-myc by Huwe1 is essential for reestablishing homeostasis following stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schuettpelz, L.G. & Link, D.C. Regulation of hematopoietic stem cell activity by inflammation. Front. Immunol. 4, 204 (2013).
Pietras, E.M., Warr, M.R. & Passegué, E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 195, 709–720 (2011).
Rossi, L. et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell 11, 302–317 (2012).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Dang, C.V. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 19, 1–11 (1999).
Scognamiglio, R. et al. Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164, 668–680 (2016).
Laurenti, E. et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell 3, 611–624 (2008).
Wilson, A. et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18, 2747–2763 (2004).
Sears, R. et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514 (2000).
Lutterbach, B. & Hann, S.R. Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol. Cell. Biol. 14, 5510–5522 (1994).
Sjostrom, S.K., Finn, G., Hahn, W.C., Rowitch, D.H. & Kenney, A.M. The Cdk1 complex plays a prime role in regulating N-myc phosphorylation and turnover in neural precursors. Dev. Cell 9, 327–338 (2005).
Gregory, M.A., Qi, Y. & Hann, S.R. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J. Biol. Chem. 278, 51606–51612 (2003).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. USA 101, 9085–9090 (2004).
Reavie, L. et al. Regulation of hematopoietic stem cell differentiation by a single ubiquitin ligase-substrate complex. Nat. Immunol. 11, 207–215 (2010).
Thompson, B.J. et al. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J. Exp. Med. 205, 1395–1408 (2008).
Matsuoka, S. et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 22, 986–991 (2008).
Zhong, Q., Gao, W., Du, F. & Wang, X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085–1095 (2005).
Adhikary, S. et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 123, 409–421 (2005).
Zhao, X. et al. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat. Cell Biol. 10, 643–653 (2008).
Zhao, X. et al. The N-Myc-DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain. Dev. Cell 17, 210–221 (2009).
Lara-Astiaso, D. et al. Immunogenetics. Chromatin state dynamics during blood formation. Science 345, 943–949 (2014).
Van Zant, G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J. Exp. Med. 159, 679–690 (1984).
Essers, M.A. et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Stadtfeld, M. & Graf, T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development 132, 203–213 (2005).
Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 238, 37–46 (2010).
Huang, C.Y., Bredemeyer, A.L., Walker, L.M., Bassing, C.H. & Sleckman, B.P. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. Eur. J. Immunol. 38, 342–349 (2008).
King, B. et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell 153, 1552–1566 (2013).
Ehninger, A. et al. Posttranscriptional regulation of c-Myc expression in adult murine HSCs during homeostasis and interferon-α-induced stress response. Blood 123, 3909–3913 (2014).
Pinto do O, P., Kolterud, A. & Carlsson, L. Expression of the LIM-homeobox gene LH2 generates immortalized steel factor-dependent multipotent hematopoietic precursors. EMBO J. 17, 5744–5756 (1998).
Hao, Z. et al. The E3 ubiquitin ligase Mule acts through the ATM-p53 axis to maintain B lymphocyte homeostasis. J. Exp. Med. 209, 173–186 (2012).
Inoue, S. et al. Mule/Huwe1/Arf-BP1 suppresses Ras-driven tumorigenesis by preventing c-Myc/Miz1-mediated down-regulation of p21 and p15. Genes Dev. 27, 1101–1114 (2013).
Jang, E.R. et al. HUWE1 is a molecular link controlling RAF-1 activity supported by the Shoc2 scaffold. Mol. Cell. Biol. 34, 3579–3593 (2014).
de Groot, R.E. et al. Huwe1-mediated ubiquitylation of dishevelled defines a negative feedback loop in the Wnt signaling pathway. Sci. Signal. 7, ra26 (2014).
Chen, D. et al. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell 121, 1071–1083 (2005).
Ivanova, N.B. et al. A stem cell molecular signature. Science 298, 601–604 (2002).
Tesio, M. & Trumpp, A. Breaking the cell cycle of HSCs by p57 and friends. Cell Stem Cell 9, 187–192 (2011).
Liu, Y. et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4, 37–48 (2009).
de Graaf, C.A. & Metcalf, D. Thrombopoietin and hematopoietic stem cells. Cell Cycle 10, 1582–1589 (2011).
Phelan, J.D. et al. Growth factor independent-1 maintains Notch1-dependent transcriptional programming of lymphoid precursors. PLoS Genet. 9, e1003713 (2013).
Satoh, Y. et al. The Satb1 protein directs hematopoietic stem cell differentiation toward lymphoid lineages. Immunity 38, 1105–1115 (2013).
Ye, M. & Graf, T. Early decisions in lymphoid development. Curr. Opin. Immunol. 19, 123–128 (2007).
Yin, L., Joshi, S., Wu, N., Tong, X. & Lazar, M.A. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erb alpha. Proc. Natl. Acad. Sci. USA 107, 11614–11619 (2010).
Herold, S. et al. Miz1 and HectH9 regulate the stability of the checkpoint protein, TopBP1. EMBO J. 27, 2851–2861 (2008).
Ye, M. et al. C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat. Cell Biol. 15, 385–394 (2013).
Riddell, J. et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 157, 549–564 (2014).
Lin, C.Y. et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151, 56–67 (2012).
Barrett, J., Birrer, M.J., Kato, G.J., Dosaka-Akita, H. & Dang, C.V. Activation domains of L-Myc and c-Myc determine their transforming potencies in rat embryo cells. Mol. Cell. Biol. 12, 3130–3137 (1992).
Malynn, B.A. et al. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 14, 1390–1399 (2000).
Metzger, M.B. & Weissman, A.M. Working on a chain: E3s ganging up for ubiquitylation. Nat. Cell Biol. 12, 1124–1126 (2010).
Farrell, A.S. & Sears, R.C. MYC degradation. Cold Spring Harb. Perspect. Med. 4, a014365 (2014).
Knoepfler, P.S., Cheng, P.F. & Eisenman, R.N. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 16, 2699–2712 (2002).
Kühn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Lakso, M. et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93, 5860–5865 (1996).
Holmes, R. & Zúñiga-Pflücker, J.C. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb. Protoc. 2009, t5156 (2009).
Anders, S. et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc. 8, 1765–1786 (2013).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Tsirigos, A., Haiminen, N., Bilal, E. & Utro, F. GenomicTools: a computational platform for developing high-throughput analytics in genomics. Bioinformatics 28, 282–283 (2012).
Ramirez, F., Dundar, F., Diehl, S., Gruning, B.A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–191 (2012).
Acknowledgements
We thank the members of the Aifantis laboratory for discussions; A. Heguy and members of the New York University (NYU) Genome Technology Center for assistance in RNA sequencing; the NYU Flow Cytometry facility for cell sorting; the NYU Histology Core; G. Inghirami for assistance with histopathological evaluations; S. Heimfeld (Fred Hutchinson Cancer Research Center) for human CD34+ cells (Core Center of Excellence NIDDK grant DK56465). Supported by the US National Institutes of Health (1R01CA169784, 1R01CA133379, 1R01CA105129, 1R01CA149655 and 5R01CA173636), the William Lawrence and Blanche Hughes Foundation, The Leukemia & Lymphoma Society (TRP#6340-11, LLS#6373-13), The Chemotherapy Foundation, The V Foundation for Cancer Research, the Alex's Lemonade Stand Foundation for Childhood Cancer, and the St. Baldrick's Cancer Research Foundation (all for the The Aifantis laboratory); the Damon Runyon Cancer Research Foundation (Berger Foundation Fellowship DRG-2234-15 to B.K.); Deutsche Forschungsgemeinschaft (Emmy Noether Research Group WO 2108/1-1 to E.W.); and the American-Italian Cancer Foundation (Alessandro and Catherine di Montezemolo endowment fund to F.B.).
Author information
Authors and Affiliations
Contributions
B.K. and I.A. designed the study and prepared the manuscript. B.K. performed most of the experiments. F.B. completed experiments and focused on N-myc genomic and transcriptomic studies. K.M.-C. initiated the Huwe1 cKO in vivo analysis. E.W. performed the N-myc ChIP-Seq. F.B. and B.A.-O. analyzed the MYCN ChIP-Seq data. J.W. and C.K. were responsible for animal husbandry. C.L. provided bioinformatics analysis and guidance. X.Y. designed the Mycn mCherry targeting vector. A.L. provided Huwe1-floxed mice and helped with data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence.
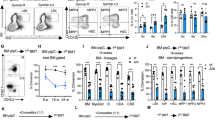
(a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin mediated proteolysis) in sorted hematopoietic populations (GSE60101), ranked by expression in HSC (LT- and ST-). (b) Huwe1 RNA-seq counts per million in hematopoietic cell populations shown in (a). (c) Frequency of HSC in bone marrow of Huwe1+/Y Mx1-Cre+ (WT) or Huwe1F/Y Mx1-Cre+ (cKO) 4 weeks post-pI:pC treatment. (d) HSPCs were sorted from bone marrow from WT or cKO mice 4 weeks post pI:pC treatment and serial colony formation in methylcellulose cultures was scored over two passages. Frequency of donor cells within total bone marrow (e) or LSK population (f) of lethally-irradiated CD45.1+ recipient mice transplanted with 1x106 bone marrow cells from untreated CD45.2+ WT or cKO mice, analyzed 28 weeks after pI:pC treatment. (g) WT or Mx1-cKO mice were given a single dose of 5-FU and cell cycle distribution was determined by intracellular Ki67/DAPI staining within gated HSC. *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed t-test). Data are representative of two experiments with five mice per group (c; mean and s.e.m.), two experiments with three technical replicates each (d; mean and s.e.m.), one experiment with four recipient mice per group (e-f; mean and s.e.m.) or one experiment with five mice per group (g; mean and s.e.m.).
Supplementary Figure 2 Huwe1-deficient fetal liver HSCs are not reduced in number but are functionally impaired.
(a) Flow cytometry of fetal livers from Huwe1+/Y Vav1-Cre+ (WT) and Huwe1F/Y Vav1-Cre+ (cKO) at E18.5 showing average frequency of Lin-c-kit+Sca1+ HSPCs (upper panels), sub-fractionated further with CD48 and CD150 (lower panels). (b) Total cells recovered from E18.5 WT or cKO fetal livers. (c) 2x104 cells from either WT or cKO E18.5 fetal livers were plated in methycellulose, scored for colony formation and harvested 7d later. 5x103 cells were replated and scored again the following week. *P < 0.05, **P < 0.01 (one-way ANOVA). Data are representative of two experiments with a minimum of three embryos per genotype (a-b; mean and s.e.m.) or three technical replicates from two embryos per genotype (c).
Supplementary Figure 3 Huwe1 is required for lymphoid specification of HSPCs in vitro.
(a) Thymii isolated from 8-week-old Huwe1+/Y Vav1-Cre+ (WT) or Huwe1F/Y Vav1-Cre+ (cKO) mice. Sorted Lin-Sca1+c-kit+ cells from the bone marrow of WT or cKO mice were co-cultured with OP9 stromal cell lines expressing either empty vector (OP9-MIG) (b) or a cDNA to ectopically express the Notch ligand Dll1 (delta-like 1) (OP9-DL1) (c). Under these conditions, bone marrow progenitors will differentiate into B cells and T cells, respectively, in the presence of Flt3-L (5 ng/ml) and IL-7 (1 ng/ml). Cells derived from either genotype were harvested at the time points shown, stained for markers of myeloid (Gr1, CD11b), B cell (CD19) and T cell (CD4, CD8, CD25, CD44) differentiation and analyzed by flow cytometry. *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed t-test). Data are representative of two experiments with three technical replicates per genotype (b-c; mean and s.e.m.).
Supplementary Figure 4 Aged Huwe1 Vav1-cKO mice exhibit myeloid expansion and anemia.
Complete blood counts (CBC) were measured from 4 month old Huwe1+/Y Vav1-Cre+ (WT) or Huwe1F/Y Vav1-Cre+ (cKO) littermates. (a) Hemoglobin (Hb) content, (b) red blood cell (RBC) counts and (c) white blood cell (WBC) counts from peripheral blood of aged WT and cKO mice are shown. (d) Light micrographs of stained blood smears (left, 20x) and histological sections of bone marrow (middle, 10x) and spleen (right, 5x) comparing tissues from WT (upper panels) and cKO (lower panels) mice. Insets are of light micrographs taken at 63x magnification. (e) Peripheral blood mononuclear cells (PBMCs) and spleen suspensions (f) from aged WT or cKO mice were analyzed for expression of mature cell markers by FACS. Average frequency of B cells (B220+), T cells (CD4+ helper or CD8+ cytotoxic) and granulocytes/monocytes (CD11b+ Gr1lo/hi) in each organ by cohort is shown. *P < 0.05, **P < 0.01 (two-tailed t-test). Data shown represents analyses of nine WT and six cKO mice (a-c, e-f; mean and s.e.m.).
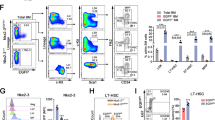
Supplementary Figure 5 Unique gene-expression signatures in N-mychi HSCs versus N-myclo HSCs.
(a) Schematic representing targeting strategy for MycnM allele. The 3 exons of Mycn, mCherry cDNA and loxP-flanked Neomycin resistance cassette are depicted. Recombination between the endogenous Mycn locus and the long (5.6kb) and short (2kb) homologous arms off the targeting construct yields MycnMNeo. Expression of Cre recombinase leads to looping out of the Neo cassette and results in a functional MycnM allele. mCherryhi and mCherrylo CD150+ HSPCs were sorted from pooled bone marrow from MycnM/M mice. Whole RNA was isolated from either population and amplified cDNA was hybridized to Affymetrix 430 2.0 microarrays. (b) Heat map of genes that were differentially expressed (fold change > 2, P < 0.05) between the N-mychi and N-myclo cells. Gene sets were tested for enrichment in expression among either population. Enrichment plots for two gene sets that were highly enriched in the N-mychi HSPCs are shown: (c) Genes upregulated in small cell lung carcinoma where MYCN is amplified and (d) Genes highly expressed in stem cells from adult tissues.
Supplementary Figure 6 Identification of genome-wide transcriptional targets of N-myc in HSCs.
(a) Smear plot illustrating global gene expression changes in Huwe1-deficient HSCs. Differentially expressed transcripts are highlighted in red. (b) Chart showing distribution of N-myc peaks across genomic regions. (c) Heat map of ChIP-sequencing read densities for N-Myc, H3K27ac, H3K4me3 and H3K27me3. All heatmaps are centered on N-myc peaks +/- 5kb and scaled to reads per million.
Supplementary Figure 7 Restoration of HSC function in Huwe1- and N-myc-dKO mice.
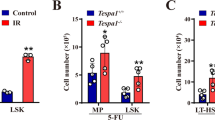
(a) HSPCs sorted from bone marrow of Huwe1+/YMycn+/+Mx1-Cre+ (WT), Huwe1F/YMycn+/+Mx1-Cre+ (Huwe1 cKO), Huwe1+/YMycnF/FMx1-Cre+ (N-myc cKO) or Huwe1F/YMycnF/FMx1-Cre+ (dKO) mice two weeks after pI:pC treatment were plated in complete methylcellulose medium (M3434) and colonies were enumerated, harvested and replated every 7d for 3 passages. (b) Absolute number of phenotypic HSC as determined by FACS in the bone marrow from mice with indicated genotypes. (c) Representative FACS histograms showing GFP fluorescence in HSC and myeloid progenitors from Mycn+/+MycG/+Mx1-Cre+ or MycnF/FMycG/+Mx1-Cre+ mice 2 weeks after pI:pC administratrion. (d) Relative levels of Mycn and Myc mRNA were measured by qRT-PCR in Lin-Kit+Sca1+ cells from bone marrow of WT or N-myc cKO mice, using Gapdh as an internal control. (e) HSPCs from Huwe1F/Y Cre- mice were transduced simultaneously with Cre (or empty) retrovirus with a bicistronic Thy.1.1 reporter and a retroviral shRNA GFP construct targeting a previously identified Huwe1 substrate or Renilla luciferase. Thy1.1+GFP+ cells were sorted 48h later, plated in methylcellulose medium and scored for colony formation as in (a). *P < 0.05, **P < 0.01 (a, e; one-way ANOVA, b,d; two-tailed t-test). Data are representative of two experiments with three technical replicates (a, e; mean and s.e.m.), analyses of four mice per genotype (b; mean and s.e.m.), or one experiment with three biological replicates (c-d; mean and s.e.m. in d).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1 and 2 (PDF 1247 kb)
Rights and permissions
About this article
Cite this article
King, B., Boccalatte, F., Moran-Crusio, K. et al. The ubiquitin ligase Huwe1 regulates the maintenance and lymphoid commitment of hematopoietic stem cells. Nat Immunol 17, 1312–1321 (2016). https://doi.org/10.1038/ni.3559
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3559
This article is cited by
-
Synergistic effect of HDAC inhibitor Chidamide with Cladribine on cell cycle arrest and apoptosis by targeting HDAC2/c-Myc/RCC1 axis in acute myeloid leukemia
Experimental Hematology & Oncology (2023)
-
TAL1 hijacks MYCN enhancer that induces MYCN expression and dependence on mevalonate pathway in T-cell acute lymphoblastic leukemia
Leukemia (2023)
-
Meloxicam with Filgrastim may Reduce Oxidative Stress in Hematopoietic Progenitor Cells during Mobilization of Autologous Peripheral Blood Stem Cells in Patients with Multiple Myeloma
Stem Cell Reviews and Reports (2021)
-
Differential Expression of Genes for Ubiquitin Ligases in Medulloblastoma Subtypes
The Cerebellum (2019)
-
Molecular switch from MYC to MYCN expression in MYC protein negative Burkitt lymphoma cases
Blood Cancer Journal (2019)