Abstract
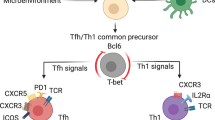
During unresolved infections, some viruses escape immunological control and establish a persistant reservoir in certain cell types, such as human immunodeficiency virus (HIV), which persists in follicular helper T cells (TFH cells), and Epstein-Barr virus (EBV), which persists in B cells. Here we identified a specialized group of cytotoxic T cells (TC cells) that expressed the chemokine receptor CXCR5, selectively entered B cell follicles and eradicated infected TFH cells and B cells. The differentiation of these cells, which we have called 'follicular cytotoxic T cells' (TFC cells), required the transcription factors Bcl6, E2A and TCF-1 but was inhibited by the transcriptional regulators Blimp1, Id2 and Id3. Blimp1 and E2A directly regulated Cxcr5 expression and, together with Bcl6 and TCF-1, formed a transcriptional circuit that guided TFC cell development. The identification of TFC cells has far-reaching implications for the development of strategies to control infections that target B cells and TFH cells and to treat B cell–derived malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
Gene Expression Omnibus
References
Mueller, S.N., Gebhardt, T., Carbone, F.R. & Heath, W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013).
Vinuesa, C.G., Linterman, M.A., Yu, D. & MacLennan, I.C. Follicular helper T cells. Annu. Rev. Immunol. 34, 335–368 (2016).
Barton, E., Mandal, P. & Speck, S.H. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu. Rev. Immunol. 29, 351–397 (2011).
Lindqvist, M. et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 122, 3271–3280 (2012).
Petrovas, C. et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 122, 3281–3294 (2012).
Perreau, M. et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 210, 143–156 (2013).
Pissani, F. & Streeck, H. Emerging concepts on T follicular helper cell dynamics in HIV infection. Trends Immunol. 35, 278–286 (2014).
Banga, R. et al. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 22, 754–761 (2016).
Kaech, S.M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Chang, J.T., Wherry, E.J. & Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 15, 1104–1115 (2014).
Wherry, E.J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Paley, M.A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Kao, C. et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12, 663–671 (2011).
Masopust, D. & Schenkel, J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 13, 309–320 (2013).
Griffith, J.W., Sokol, C.L. & Luster, A.D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702 (2014).
Sallusto, F. Heterogeneity of human CD4+ T cells against microbes. Annu. Rev. Immunol. 34, 317–334 (2016).
Vinuesa, C.G. & Cyster, J.G. How T cells earn the follicular rite of passage. Immunity 35, 671–680 (2011).
Weinmann, A.S. Regulatory mechanisms that control T-follicular helper and T-helper 1 cell flexibility. Immunol. Cell Biol. 92, 34–39 (2014).
Hong, J.J., Amancha, P.K., Rogers, K., Ansari, A.A. & Villinger, F. Spatial alterations between CD4+ T follicular helper, B, and CD8+ T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J. Immunol. 188, 3247–3256 (2012).
Fukazawa, Y. et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 21, 132–139 (2015).
Quigley, M.F., Gonzalez, V.D., Granath, A., Andersson, J. & Sandberg, J.K. CXCR5+ CCR7− CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol. 37, 3352–3362 (2007).
Zhou, X., Ramachandran, S., Mann, M. & Popkin, D.L. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses 4, 2650–2669 (2012).
Kyburz, D. et al. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 23, 1956–1962 (1993).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Homann, D., McGavern, D.B. & Oldstone, M.B. Visualizing the viral burden: phenotypic and functional alterations of T cells and APCs during persistent infection. J. Immunol. 172, 6239–6250 (2004).
Baca Jones, C. et al. Direct infection of dendritic cells during chronic viral infection suppresses antiviral T cell proliferation and induces IL-10 expression in CD4 T cells. PLoS One 9, e90855 (2014).
Oxenius, A., Bachmann, M.F., Zinkernagel, R.M. & Hengartner, H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28, 390–400 (1998).
Afshar-Sterle, S. et al. Fas ligand-mediated immune surveillance by T cells is essential for the control of spontaneous B cell lymphomas. Nat. Med. 20, 283–290 (2014).
Gaspar, M. et al. Murid herpesvirus-4 exploits dendritic cells to infect B cells. PLoS Pathog. 7, e1002346 (2011).
Kim, H.J., Verbinnen, B., Tang, X., Lu, L. & Cantor, H. Inhibition of follicular T-helper cells by CD8+ regulatory T cells is essential for self tolerance. Nature 467, 328–332 (2010).
Johnston, R.J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Miyazaki, M. et al. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat. Immunol. 12, 992–1001 (2011).
Choi, Y.S. et al. LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 16, 980–990 (2015).
Wu, T. et al. TCF1 is required for the T follicular helper cell response to viral infection. Cell Reports 12, 2099–2110 (2015).
Xu, L. et al. The transcription factor TCF-1 initiates the differentiation of TFH cells during acute viral infection. Nat. Immunol. 16, 991–999 (2015).
Shaw, L.A. et al. Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat. Immunol. 17, 834–843 (2016).
Xin, A. et al. A combinatorial threshold model for effector differentiation of CD8+ T cells mediated by Blimp-1 and T-bet. Nat. Immunol. 17, 422–432 (2016).
Chen, Y. & Yu, D. TCF-1 at the Tfh and Th1 Divergence. Trends Immunol. 36, 758–760 (2015).
Ji, Y. et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat. Immunol. 12, 1230–1237 (2011).
Masson, F. et al. Id2-mediated inhibition of E2A represses memory CD8+ T cell differentiation. J. Immunol. 190, 4585–4594 (2013).
Hatzi, K. et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med. 212, 539–553 (2015).
Liu, X. et al. Genome-wide analysis identifies Bcl6-controlled regulatory networks during T follicular helper cell differentiation. Cell Rep. 14, 1735–1747 (2016).
Shaffer, A.L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Oestreich, K.J., Mohn, S.E. & Weinmann, A.S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 13, 405–411 (2012).
Kallies, A., Xin, A., Belz, G.T. & Nutt, S.L. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009).
Rutishauser, R.L. et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Mackay, L.K. et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016).
Junying, J. et al. Absence of Epstein-Barr virus DNA in the tumor cells of European hepatocellular carcinoma. Virology 306, 236–243 (2003).
Hollister, K. et al. Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J. Immunol. 191, 3705–3711 (2013).
Steinke, F.C. et al. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4+ T cell fate and interact with Runx3 to silence Cd4 in CD8+ T cells. Nat. Immunol. 15, 646–656 (2014).
Kallies, A. et al. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J. Exp. Med. 200, 967–977 (2004).
Jackson, J.T. et al. Id2 expression delineates differential checkpoints in the genetic program of CD8α+ and CD103+ dendritic cell lineages. EMBO J. 30, 2690–2704 (2011).
Lüthje, K. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 13, 491–498 (2012).
Minnich, M. et al. Multifunctional role of the transcription factor Blimp-1 in coordinating plasma cell differentiation. Nat. Immunol. 17, 331–343 (2016).
Korns Johnson, D. & Homann, D. Accelerated and improved quantification of lymphocytic choriomeningitis virus (LCMV) titers by flow cytometry. PLoS One 7, e37337 (2012).
Hislop, A.D., Annels, N.E., Gudgeon, N.H., Leese, A.M. & Rickinson, A.B. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 195, 893–905 (2002).
Deleage, C. et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog. Immun. 1, 68–106 (2016).
Jedema, I., van der Werff, N.M., Barge, R.M., Willemze, R. & Falkenburg, J.H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood 103, 2677–2682 (2004).
Xing, S. et al. Tcf1 and Lef1 transcription factors establish CD8+ T cell identity through intrinsic HDAC activity. Nat. Immunol. 17, 695–703 (2016).
Acknowledgements
We acknowledge the facilities, scientific and technical assistance of Flowcore, Monash Micro Imaging, and Monash Bioinformatics Platform (S. Archer and K. Tsyganov) at Monash University, and University of Birmingham's Human Biomaterials Resource Centre (supported through Birmingham Science City - Experimental Medicine Network of Excellence project). We thank L. Ye (Third Military Medical University) for Thy1.1 reporter constructs; C. Vinuesa (Australian National University) and S. Nutt (Walter and Eliza Hall Institute of Medical Research) for mice; R. Gloury and L. Mackiewicz for technical support; C. Dong for the list of Bcl6-bound genes; and H. Xue for the list of TCF-1-bound genes. Supported by the National Health and Medical Research Council of Australia (Y.A.L. and S.R.L.; GNT1085509 to D.Y.; and GNT1085151 to A.K.), Monash University (D.Y.), the amfAR Research Consortium on HIV Eradication (109327-59-RGRL, D.Y., S.R.L. and A.L.L.), The Creative and Novel Ideas in HIV Research Program of The International AIDS Society (D.Y.), Australian Centre for HIV and Hepatitis Virology Research (2015-69 to D.Y.), The Priority Research Program of Shandong Academy of Sciences (D.Y.), Shandong Province Taishan Scholar Program (D.Y.), the Sylvia and Charles Viertel Foundation (A.K.), the Delaney AIDS Research Enterprise to find a cure, Martin Delaney Collaboratories, the National Institute for Allergy and Infectious Diseases of the US National Institutes of Health (U19 AI096109 to S.R.L., T.W.S. and J.D.E.), Bloodwise, UK (15021 to H.M.L. and B.J.M.), the National Cancer Institute of the US National Institutes of Health (HHSN261200800001E), and the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC Independent Research Institute Infrastructure Support scheme. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Contributions
Y.A.L. planned and performed most experiments with the support from Y.C. and H.S.O.; Y.C. and A.A. performed experiments related to retrovirus-mediated gene overexpression; D.W. and J.X. performed bioinformatics analysis; K.M. performed experiments to generate chimeric mice; C.D, J.G.C., G.J.B., T.W.S. and J.D.E. performed immunofluorescence staining and quantification of HIV samples; M.M., M.B. and A.K. performed and/or analyzed the ChIP-seq experiments; B.J.M., U.P. and H.M.L. performed analysis of EBV samples; Y.W., Z.H., L.S., P.W., and Y.T. performed flow cytometry on HIV samples; D.Z. and A.K. performed experiments on lymphoma; K.A.F., I.C. and S.R.M. performed experiments on influenza infection; S.P., C.C.A., J.G.T. and M.P. provided support for experiments on LCMV and relevant models; H.K.L. provided support for experiment to quantify viral RNA; A.L.D. provided Bcl6fl/fl mice; A.L.L. and S.R.L. contributed to scientific planning; G.T.B. provided support on the MuHV-4 model; D.Y. and A.K. oversaw and designed the study; Y.A.L. and D.Y. analyzed data; and Y.A.L., A.K. and D.Y. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 CXCR5-expressing TFC cells are generated in immune responses and are localized in B cell follicles.
(a) Staining of anti-CD3 (red), anti-CD8 (green) and anti-B220 (blue) of splenic sections from uninfected mice or mice at day 8 post-infection (p.i.) of LCMV. CD8+ T cells (yellow) indicated by higher magnification of B cell follicles. Data are representative of 2 independent experiments.
(b) Congenically marked CD45.1+ P14 cells were adoptively transferred into CD45.2+ wildtype mice, which were then infected with LCMV (DOCILE). Flow cytometric analysis of CCR7 expression on P14 cells (red box) or endogenous activated CD44+ TC cells (blue box) at day 8 p.i.
(c) CD45.1 CXCR5+/+ or CXCR5−/− P14 cells were adoptively transferred into congenically marked (CD45.2) wildtype mice, which were then infected with LCMV (DOCILE). Flow cytometric analysis of CXCR5 expression on P14 cells at day 8 p.i..Numbers adjacent to each outlined area indicate percentage of population. **P < 0.01 (Mann Whitney's U-test)
(d, e) OT-I cells (CD45.2) were adoptively transferred into congenically marked (CD45.1) wildtype mice, which were then intravenously infected with OVA-expressing influenza virus (d) or immunized subcutaneously at hock with OVA in CFA. Flow cytometric analysis of CXCR5 expression on OT-I cells in in spleens at day 10 p.i (d) or popliteal lymph nodes at day 8 post immunization (e). Numbers adjacent to each outlined area indicate percentage of population.
Supplementary Figure 2 CD4+ T cells are significantly infected by LCMV (DOCILE).
(a) Intracellular staining of LCMV nucleoprotein (NP) in splenic CD4+ T cells from uninfected, day 8 post OVA-CFA immunization, day 8 p.i. with LCMV (WE) or LCMV (Docile) mice. Each symbol represents one mouse, bars represent means. **P < 0.01 (Mann Whitney's U-test)
(b) Mice were infected with LCMV (DOCILE). At day 15 p.i., TH subsets in spleens (gated as in Fig. 4b) were purified and pooled. The LCMV viral RNA was measured by qPCR. Data are representative of two independent experiments. Each symbol represents one experimental replicate, bars represent means. **P < 0.01 (Mann Whitney's U-test)
Supplementary Figure 3 Control of TFH cell infection by TFC cells.
(a) Schematic of the experiment.
(b) Quantification of splenic TFH differentiation.
(c) Quantification of P14 cells in spleens.
(d, e) Viral loads in sera (d) and spleens (e), as measured by plague forming assays. Data are representative of two independent experiments. Each symbol represents one mouse, bars represent means. NS = not significant, P > 0.05 (Mann Whitney's U-test).
Supplementary Figure 4 Population expansion and activation of transferred CD8+ T cells in mice infected with MuHV-4.
(a) Representative FACS plots showing the total CD8 T cells with transferred cells marked by congenic marker CD45.1.
(b) Quantification of transferred cells (left) or activated transferred cells (right) in total CD8+ T cells. Data are representative of two independent experiments. Each symbol represents one mouse, bars represent means. NS, not significant, P > 0.05 (Mann Whitney's U-test).
Supplementary Figure 5 The top 50 genes up- and downregulated in TFC cells and non-TFC cells.
Heatmaps for normalized log2 data of the top 50 lists of upregulated (a) or downregulated (b) genes.
Supplementary Figure 6 The phenotype and function of TFC cells.
CD45.1+ P14 cells were adoptively transferred into congenically marked (CD45.2) wildtype mice, which were then infected with LCMV (DOCILE). The expression of indicated proteins in naïve (grey), CXCR5+ TFC (red), CXCR5- non-TFC (blue), B220+ B (black) cells at day 8 p.i.
(a) The expression of ICOSL on TFC cells.
(b) IL-21-GFP reporter mice were infected with LCMV Docile and the expression of IL-21-GFP in indicated populations were analysed at day 15 p.i..
(c) Ex vivo killing of LCMV GP33-41 peptide-pulsed non-TFH or TFH SMARTA cells, or B cells by CXCR5+ TFC or CXCR5− non-TFC P14 cells purified at day 8 p.i.
Each symbol represents one mouse (A, B and D) or one sample (C), bars represent means. GMFI: geometric mean fluorescence intensity. **P < 0.01 (Mann-Whitney's U-test). Data are representative of two independent experiments.
Supplementary Figure 7 Bcl6 regulates the phenotype and function of TC cells.
(a) Gene set enrichment test of genes differentially expressed in TFC cells as compared to non-TFC cells in differentially expressed genes in TFH cells as compared to non-TFH cells. Red and blue bars designate up and down-regulated genes in TFC cells, respectively. Correlation of up (top) and down (bottom)-regulated genes were shown by rotation gene set test P values and the percentages.
(b-e) P14 cells were transduced with a GFP retroviral empty vector (RV) or the vector expressing Bcl6.
(b, c) Transduced cells were transferred into congenically marked recipient mice which were subsequently infected with LCMV (DOCILE). The expression of indicated proteins on transduced cells was measured by flow cytometry at day 8 p.i.,
(d) Transduced cells were co-cultured with LCMV gp33–41 peptide-pulsed splenocytes and the killing of target cells were measured by flow cytometry.
(e) Transduced cells were purified and the indicated genes were measured by quantitative PCR.
*P < 0.05, **P < 0.01 (Mann Whitney's U-test). Data are representative of two independent experiments.
Supplementary Figure 8 Transcriptional regulation of TFC cell differentiation.
(a-c) Enrichment analysis of the differentially expressed genes in TFC cells. Gene set enrichment test of genes differentially expressed in TFC cells as compared to non-TFC cells in differentially expressed genes in Blimp1-deficient TC cells (a), Id3-deficient TC cells (b) and Id2-deficient TC cells (c) as compared to counterpart wildtype TC cells. Red and blue bars designate up and down-regulated genes in TFC cells, respectively. Correlation of up (top) and down (bottom)-regulated genes were shown by rotation gene set test P values and the percentages.
(d, f-h) Correlation between transcription factor-bound genes and TFC signature genes. Gene set enrichment test of genes bound by Bcl6 (d), E2A (f), Blimp1 (g) or TCF-1 (h) among transcripts differentially expressed in TFC cells versus non-TFC cells. Red and blue bars designate upregulated and downregulated genes in TFC cells, respectively. Significant correlation of binding sites (barcode plots) with differentially expressed genes is shown by P value. Percentages show proportion of genes bound by each transcription factor that were also differentially expressed in TFC vs non-TFC.
(e) Binding of Blimp1 and E2A at the indicated genes. ChIP-seq analysis of Blimp1 and E2A binding was performed with CD8+ effector T cells and total thymocytes, respectively. Binding regions, which were identified by MACS peak calling, are indicated by black rectangles below the horizontal axis. RPM; reads per million.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 2963 kb)
Rights and permissions
About this article
Cite this article
Leong, Y., Chen, Y., Ong, H. et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 17, 1187–1196 (2016). https://doi.org/10.1038/ni.3543
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3543
This article is cited by
-
Id2 epigenetically controls CD8+ T-cell exhaustion by disrupting the assembly of the Tcf3-LSD1 complex
Cellular & Molecular Immunology (2024)
-
FOXP1 and KLF2 reciprocally regulate checkpoints of stem-like to effector transition in CAR T cells
Nature Immunology (2024)
-
Regulation of CD8+ T memory and exhaustion by the mTOR signals
Cellular & Molecular Immunology (2023)
-
Splenic stromal niches in homeostasis and immunity
Nature Reviews Immunology (2023)
-
Follicular Helper T Cells and Follicular Regulatory T Cells Involved in Immune Disorders of Idiopathic Membranous Nephropathy
Indian Journal of Pediatrics (2023)