Abstract
Macrophages tightly scale their core metabolism after being activated, but the precise regulation of the mitochondrial electron-transport chain (ETC) and its functional implications are currently unknown. Here we found that recognition of live bacteria by macrophages transiently decreased assembly of the ETC complex I (CI) and CI-containing super-complexes and switched the relative contributions of CI and CII to mitochondrial respiration. This was mediated by phagosomal NADPH oxidase and the reactive oxygen species (ROS)-dependent tyrosine kinase Fgr. It required Toll-like receptor signaling and the NLRP3 inflammasome, which were both connected to bacterial viability–specific immune responses. Inhibition of CII during infection with Escherichia coli normalized serum concentrations of interleukin 1β (IL-1β) and IL-10 to those in mice treated with dead bacteria and impaired control of bacteria. We have thus identified ETC adaptations as an early immunological-metabolic checkpoint that adjusts innate immune responses to bacterial infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Blander, J.M. & Sander, L.E. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat. Rev. Immunol. 12, 215–225 (2012).
Taylor, P.R. et al. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944 (2005).
Huang, S.C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855 (2014).
Jha, A.K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Rodríguez-Prados, J.C. et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 (2010).
Tannahill, G.M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
O'Neill, L.A. & Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Stanley, I.A., Ribeiro, S.M., Giménez-Cassina, A., Norberg, E. & Danial, N.N. Changing appetites: the adaptive advantages of fuel choice. Trends Cell Biol. 24, 118–127 (2014).
Weinberg, S.E., Sena, L.A. & Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 42, 406–417 (2015).
Moon, J.S. et al. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep. 12, 102–115 (2015).
Michelucci, A. et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 110, 7820–7825 (2013).
Naujoks, J. et al. IFNs modify the proteome of legionella-containing vacuoles and restrict infection via IRG1-derived itaconic acid. PLoS Pathog. 12, e1005408 (2016).
West, A.P., Shadel, G.S. & Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 11, 389–402 (2011).
Enríquez, J.A. Supramolecular organization of respiratory complexes. Annu. Rev. Physiol. 78, 533–561 (2016).
Schägger, H. & Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19, 1777–1783 (2000).
Kelly, B., Tannahill, G.M., Murphy, M.P. & O'Neill, L.A. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 290, 20348–20359 (2015).
Jin, Z., Wei, W., Yang, M., Du, Y. & Wan, Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 20, 483–498 (2014).
Sander, L.E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011).
Lapuente-Brun, E. et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570 (2013).
Acín-Pérez, R., Fernández-Silva, P., Peleato, M.L., Pérez-Martos, A. & Enriquez, J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell 32, 529–539 (2008).
Speijer, D. Oxygen radicals shaping evolution: why fatty acid catabolism leads to peroxisomes while neurons do without it: FADH/NADH flux ratios determining mitochondrial radical formation were crucial for the eukaryotic invention of peroxisomes and catabolic tissue differentiation. BioEssays 33, 88–94 (2011).
Guarás, A. et al. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 15, 197–209 (2016).
Benard, G. et al. Functional dynamic compartmentalization of respiratory chain intermediate substrates: implications for the control of energy production and mitochondrial diseases. Int. J. Biochem. Cell Biol. 40, 1543–1554 (2008).
Mrácček, T., Drahota, Z. & Houštěk, J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta 1827, 401–410 (2013).
West, A.P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Kelso, G.F. et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 276, 4588–4596 (2001).
Nunes, P., Demaurex, N. & Dinauer, M.C. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic 14, 1118–1131 (2013).
Acín-Pérez, R. et al. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab. 19, 1020–1033 (2014).
Nath, A.K. et al. PTPMT1 inhibition lowers glucose through succinate dehydrogenase phosphorylation. Cell Rep. 10, 694–701 (2015).
Lowell, C.A. Src-family kinases: rheostats of immune cell signaling. Mol. Immunol. 41, 631–643 (2004).
Alston, T.A., Mela, L. & Bright, H.J. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc. Natl. Acad. Sci. USA 74, 3767–3771 (1977).
Gutman, M. Modulation of mitochondrial succinate dehydrogenase activity, mechanism and function. Mol. Cell. Biochem. 20, 41–60 (1978).
Pan, H. & Wu, X. Hypoxia attenuates inflammatory mediators production induced by Acanthamoeba via Toll-like receptor 4 signaling in human corneal epithelial cells. Biochem. Biophys. Res. Commun. 420, 685–691 (2012).
Vance, R.E., Isberg, R.R. & Portnoy, D.A. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6, 10–21 (2009).
Kanneganti, T.D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006).
Sokolovska, A. et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat. Immunol. 14, 543–553 (2013).
Acín-Pérez, R. et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol. Cell 13, 805–815 (2004).
Diaz, F., Fukui, H., Garcia, S. & Moraes, C.T. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol. Cell. Biol. 26, 4872–4881 (2006).
Huang, Y.L. et al. Toll-like receptor agonists promote prolonged triglyceride storage in macrophages. J. Biol. Chem. 289, 3001–3012 (2014).
Geng, J. et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol. 16, 1142–1152 (2015).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267, 2000–2003 (1995).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Drutman, S.B. & Trombetta, E.S. Dendritic cells continue to capture and present antigens after maturation in vivo. J. Immunol. 185, 2140–2146 (2010).
Blander, J.M. & Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004).
Schägger, H. & von Jagow, G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199, 223–231 (1991).
Wittig, I., Braun, H.P. & Schägger, H. Blue native PAGE. Nat. Protoc. 1, 418–428 (2006).
Birch-Machin, M.A. & Turnbull, D.M. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 65, 97–117 (2001).
Vives-Bauza, C., Yang, L. & Manfredi, G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 80, 155–171 (2007).
Acknowledgements
We thank A. Hidalgo for critical reading of the manuscript; S. Akira (Osaka University) for Ips1−/− mice; J. Magarian Blander (Mount Sinai School of Medicine) for Myd88−/−, Trif−/− and Trif−/−Myd88−/− mice; F. Norel-Bozouklian (Institut Pasteur) for S. thyphimurium SL1344; S. Trombetta (New York University) for the plasmid encoding GFP-OT; S. Bartlett for English editing; and M. Fernández-Monreal, M. Villalba, F. Ruperez-Pascualena and members of the Sancho and Enríquez laboratories for discussions and support. Supported by the European Community (FP7-Marie Curie-CIG#332881 to J.G.; ERC-2010-StG 260414 and 635122-PROCROP H2020 to D.S.; and UE0/MCA1108 and UE0/MCA1201 to J.A.E.), the French association 'La Ligue Contre le Cancer-comité du Gard' (CG/59-2013 to J.G.), the Spanish Ministry of Economy and Competitiveness (SAF-2013-42920R to D.S.; SAF2012-1207 to J.A.E.; and RyC2011-07826 to R.A.-P.), the Comunidad de Madrid (CAM/API1009 to J.A.E.), the German research council (DFG grant SA1940/2-1 and SFB-TR84 TP-C08 to L.E.S.). CNIC is supported by the MINECO and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
Author information
Authors and Affiliations
Contributions
J.G. and R.A.-P. designed and performed all experiments; S.M.-C. and M.E. performed experiments measuring oxidative-phosphorylation enzymatic activity, prepared samples for BN-PAGE and immunoblot analysis and helped with experiments with mice; M.U. and L.E.S. performed experiments with human monocytes; E.N.-V. and S.H.-S. provided bone marrow progenitor cells from STING-deficient and MAVS-deficient mice and generated MAVS-deficient Trif−/− mice; P.P. provided Nlrp3−/− and Casp1−/−Casp11−/− mice; and J.G., R.A.-P., J.A.E. and D.S. directed the study, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Effect of E. coli challenge on expression of subunits of the mitochondrial respiratory complex in BMDMs.
(a) Densitometric analysis of the distribution of complex I, III and IV as determined from Figure 1a. (b) Gene microarray analysis (Affymetrix Microarray data have been previously deposited with the NCBI Gene Expression Omnibus under accession number GSE27960 by Sander L.E. et al., see ref. 19) of C57BL/6J BMDMs treated with E. coli for 1, 3 or 6h (3 biological replicates). A heat map of nuclear genome-encoded mitochondrial respiratory complexes subunits is shown. (c) Q-PCR analysis of the indicated gene from BMDMs treated with E. coli for 1.5h. (d) Immunoblot analysis of resting BMDMs and E. coli. (e) Immunoblot analysis of BMDMs treated as indicated for 1.5h. EC, E. coli; HKEC, heat killed-E. coli; Poly I:C, polyinosinic:polycytidylic acid; LPS, lipopolysaccharide. (f) Quantification analysis of SDS-PAGE as in (e). NS, not significant (P > 0.05); **P < 0.01 (two-tailed unpaired Student’s t-test). Membranes were probed with the indicated antibodies specific for components of the ETC (d, e). Data are from three independent experiments (c, f;mean and s.e.m.), one representative of two independent experiments with similar results (a, d, e).
Supplementary Figure 2 Detection of Gram-negative bacteria induces ETC rearrangement and is associated with decreased CI.
(a) Immunoblot of a bi-dimensional gel analysis (First dimension: BN-PAGE, second dimension: SDS-PAGE) of mitochondria isolated from C57BL/6J BMDMs treated or not with EC for 1.5h. (b) Quantification from (a) of the proportion of free CIII and CIII in super-complex with CI (SC I+III). (c) BN-PAGE immunoblot of C57BL/6J thioglycollate-elicited macrophages treated or not with EC. (d) BN-PAGE immunoblot of C57BL/6J BMDMs treated with E. coli and S. enterica Typhimurium for 1.5h. (e) Densitometric analysis of BN-PAGE from (d) showing CI+CIII SC proportion vs. CII (n=4). **P < 0.01 (two-tailed unpaired Student’s t-test). Data present mean +/- s.e.m. of 4 independent experiments. (f) CI IGA of BMDMs treated or not with E. coli or S. enterica Typhimurium for 1.5h. SDS-PAGE analysis of FpSDH (CII) is shown (lower panel). Data are from two independent experiments (e, mean and s.e.m.), one representative of two independent experiments (a-d, f).
Supplementary Figure 3 E. coli challenge influences mitochondrial respiration and glycolysis in macrophages.
(a) Glucose-driven basal respiration rate (BRR), maximum respiration rate (MRR), spare respiration capacity (SRC), basal extracellular acidification rate (ECAR) and oxygen consumption rate (OCR)/ECAR ratio in BMDMs treated or not with EC for 18h. (b) Glucose-driven OCR upon sequential treatment of oligomycin (olig.), CCCP, and rotenone+antimycin (Rot.+Ant.) of BMDMs treated or not with EC for 18h. (c) Extracellular lactate release by BMDMs treated or not with EC for 18h. (d) Glucose-driven BRR, MRR and OCR/ECAR ratio of BMDMs treated or not with EC for 1.5h. NS, not significant, ***P < 0.001 (two-tailed unpaired Student’s t-test). Data (means and s.d.) are from one representative of two independent experiments (a, b). Data (means and s.e.m.) are from three independent experiments performed in five technical replicates (c, d).
Supplementary Figure 4 Recognition of bacteria drives mitochondrial respiratory adaptations through phagosomal NADPH oxidase–mediated ROS.
(a) Representative histograms of BMDMs treated as indicated for 1.5h, stained with mitoSOX (mROS) and analysed by FACS. Rot., rotenone. (b) mROS production by BMDMs treated as in (a). One representative experiment performed in triplicate is shown. (c-e) ROS production by EC alone (c), WT BMDMs (c-e) and Gp91phox-/- BMDMs stimulated or not with EC for 15min (e). ROS production was monitored by chemiluminescence and expressed as relative light units per second (R.L.U./s). In (d), BMDMs were treated with the CII inhibitors 3-nitropropionic acid (NPA), dimethyl-malonate (DM) or thenoyltrifluoroacetone (TTFA). (f) Glucose-driven OCR upon sequential treatment of oligomycin (olig.), CCCP, and rotenone+antimycin (Rot.+Ant.) of WT and Gp91phox-/- BMDMs challenged with EC for 2h. (g) Maximum (MRR) and basal (BRR) respiration rate of WT and Gp91phox-/- BMDMs challenged with EC for 2h. ***P < 0.001 (two-tailed unpaired Student’s t-test (c, d, e)). Data (mean and s.e.m.) are from three independent experiments performed in duplicates (c-e), one representative of three independent experiments. Data are mean and s.d. of one representative of three independent experiments performed in three (a, b) and five (f, g) technical replicates.
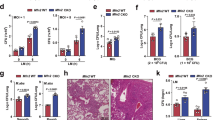
Supplementary Figure 5 Fgr deficiency prevents macrophage ETC adaptations and attenuates innate immunity in response to detection of E. coli.
(a) Immunoblot analysis of BMDMs stimulated as indicated for 1.5h and probed with the indicated antibodies specific for component of the ETC. (b) Percentage of phagocytic cells of WT and Fgr-/- BMDMs treated with NPA and cultured with GFP-expressing E. coli (E. coli-GFP) or FITC-labeled latex beads (Beads-FITC) for 20min. (c, d) Spectrophotometric CII activity in permeabilized Fgr-/- BMDMs stimulated with EC for 1.5h or the indicated time points and treated or not with N-acetylcystein (NAC) or mitoQ. (e) Immunoblot of BN-PAGE analysis of permeabilized Fgr-/- BMDMs stimulated as indicated for 1.5h. (f) Immunoblot of a bi-dimensional gel analysis (BN-PAGE followed by a SDS-PAGE) of mitochondrial isolated from Fgr-/- BMDMs treated or not with EC for 1.5h. (g) Quantification from (f) of the proportion of CI and CIII as free form or in super-complex (SC I+III). (h) Immunoblot of BN-PAGE analysis of mitochondrial isolated from WT and Fgr-/- BMDMs stimulated as indicated for 1.5h. Lower panel shows CI in-gel activity (IGA). (i) Relative contribution of CIII to SC as determined by BN-PAGE analysis of mitochondria isolated from Fgr-/- and WT BMDMs. (j) Effect of E. coli stimulation on the indicated ETC complex activities in mitochondria isolated from WT and Fgr-/- BMDMs. (k) Glucose-driven OCR upon sequential treatment of oligomycin (olig.), CCCP, and rotenone+antimycin (Rot.+Ant.) of WT and Fgr-/- BMDMs stimulated or not with EC for 2h. (l) Splenic bacterial burdens 72h after injection of 1 x 108 of viable E. coli into the peritoneal cavity of WT and Fgr-/- mice. Each symbol represents one mouse. (m) Absolute cell numbers determined at 18h by FACS of the indicated cell populations in the peritoneal cavity of WT and Fgr-/- mice injected with 1x108 viable EC. (n) mRNA levels in WT and Fgr-/- BMDMs stimulated with EC for the indicated time point. (o) Cytokine levels in supernatants of WT and Fgr-/- BMDMs stimulated with EC for 18h. NS, not significant; *P < 0.05, ***P<0.001 (two-tailed unpaired Student’s t-test). Data (mean and s.e.m.) are from two to four independent experiments performed in two or five technical replicates (b-d, j, l-o). Data (k, means and s.d.; a, e, f, h) are from one representative of three independent experiments performed in five technical replicates. EC, E. coli; HKEC, heat killed-E. coli; pI:C, polyinosinic:polycytidylic acid; LPS, lipopolysaccharide.
Supplementary Figure 6 Effects of inhibition of mitochondrial CII on BMDM function and mitochondrial respiration.
(a) Percentage of live BMDMs treated with 0.5mM NPA and challenged with EC for 1.5h. Cells were stained with annexin-V-GFP and 7-aminoactinomycin D (7-AAD) and analyzed by fluorescent-activated cell sorting flow cytometry (FACS). Live cells were defined as annexin-V-/7-AAD- cells. (b) Percentage of phagocytic cells (right panel) of BMDMs treated with 0.5mM NPA and cultured with GFP-expressing E. coli (E. coli-GFP) or FITC-labeled latex beads (Beads-FITC) for 20min. Representative FACS plots (left panel) are shown. (c) Spectrophotometric CII activity of BMDMs treated or not with EC in presence of 0.5mM NPA. (d) Spectrophotometric SDH activity of log phase EC or heat-killed EC (HKEC) in the presence of NPA. (e) E. coli growth measured by spectrophotometry (A600) in presence of the indicated concentrations of NPA over a course of 8h. (f) BN-PAGE immunoblot or permeabilized C57BL/6J BMDMs treated or not with NPA stimulated with EC for 1.5h. Membranes were stained with the indicated antibodies. (g) Glutamate+malate (CI) or succinate (CII)-driven ATP synthesis activity in permeabilized C57BL/6J BMDMs treated or not with NPA stimulated with EC for 1.5h. (h) Glucose-driven OCR, BRR and MRR upon sequential treatment of oligomycin (olig.), CCCP, and rotenone+antimycin (Rot.+Ant.) of BMDMs treated or not with NPA and challenged with EC for 2h. (i) OCR/ECAR ratio of BMDMs treated or not with NPA and challenged with EC for 2h. (j) Extracellular lactate release by BMDMs treated or not with NPA and challenged with EC for 2h. (k) Glucose-driven OCR, BRR and MRR upon sequential treatment of oligomycin (olig.), CCCP, and rotenone+antimycin (Rot.+Ant.) of BMDMs treated or not dimethyl-malonate (DM) and challenged with EC for 2h. (l-o) SRC (l, n) and ECAR (m, o) of BMDMs treated or not with dimethyl-malonate (DM) (l, m) or thenoyltrifluoroacetone (TTFA) (n, o) and challenged with EC for 2h. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 (two-tailed unpaired Student’s t-test). Data (mean and s.e.m.) are from two independent experiments performed in two to five technical replicates (a, b, right, e, g, i, j, l-o). Data in (b, left, c, d, f, h, k) are mean and s.d. of three technical replicates from one representative of three independent experiments.
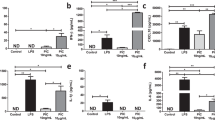
Supplementary Figure 7 CII activity is required for macrophage bactericidal function, and inhibition of CII by 3-nitropropionic acid (NPA) alters the innate immune response to viable E. coli.
(a) Absolute cell numbers at 18h (right panels) of the indicated cell populations in the peritoneal cavity of C57BL/6J mice treated or not with 50mg/kg NPA and injected with 1x108 viable EC. Representative FACS plots (left panels) are shown. (b, c) mRNA (b) and cytokine levels (c) in BMDMs treated or not with NPA and stimulated with EC for the indicated time points. (d) Representative photographs of Petri dishes containing bacteria grown overnight after treatment with the indicated chemicals for 6h. As control, heat-killed E. coli (HKEC) were plated. (e) Representative flow cytometry plots of E.coli treated with the indicated reagents for 6h. (f) Percentage of PI+SYTO+ bacteria after 6h treatment with increasing amount of the indicated reagents. NS, not significant; **P < 0.01 ***P < 0.001 (two-tailed unpaired Student’s t-test). Data (mean and s.e.m. (a,b,c,f)) are from two (c, e, f) or three (a, b) independent experiments performed in triplicates. Data in (d) are from one representative of two independent experiments with similar results.
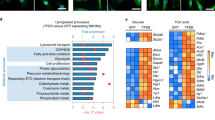
Supplementary Figure 8 Bacteria viability–specific ETC adaptations involve both TLR signaling and the Nlrp3 inflammasome.
(a) Densitometry analysis of CI+CIII2/CII signal ratio as observed by BNGE immunoblot of WT BMDMs stimulated or not with EC and HKEC for 1.5h. (b) Blue-native gel electrophoresis (BN-PAGE) immunoblot in mitochondria isolated from CD1 BMDMs stimulated with EC, HKEC or lipopolysaccharide (LPS) for 1.5h. (c) Glucose-driven OCR upon sequential treatment of oligomycin (olig.), CCCP, and rotenone+antimycin (Rot.+Ant.) of BMDMs treated or not with viable E. coli (EC) or heat-killed E. coli (HKEC) for 2h. (d) Extracellular lactate release by WT BMDMs treated with EC or HKEC for 2h. (e) Spectrophotometric mG3PDH activity in WT BMDM stimulated with viable EC or HKEC for 1.5h. (f) Spectrophotometric CII activity in permeabilized thioglycollate-elicited peritoneal macrophages (PEM) stimulated with viable EC or HKEC for 1h. (g) ROS production by WT BMDMs stimulated with EC or HKEC at the indicated multiplicity of infection (M.O.I.) for 15min. ROS production was monitored by chemiluminescence and expressed as relative light units per second (R.L.U./s). (h) Agarose gel electrophoresis of EC and HKEC total RNA before and after treatment with RNases III and A/T1. (i) IL-6 cytokine levels in supernatants of WT BMDMs treated with the indicated TLR ligand. (j) Ifnb mRNA levels in WT BMDMs stimulated with poly(I:C) for the indicated time point. (k) Spectrophotometric CII activity in permeabilized WT, Trif-/-, Mavs-/-, Trif-/-Mavs-/- and Sting deficient (Tmem173gt, here called GT-Sting) BMDMs stimulated with viable E. coli (EC) for 1.5h. (l) BNGE analysis from permeabilized WT, Trif-/-, and Trif-/-Myd88-/- BMDMs stimulated with EC for 1.5h. Arrows indicate the main SCs affected. Densitometry analysis of the SCI+III2 vs CII is shown on the right panel. (m) Representative BNGE analysis from permeabilized WT, Caspase1-/-xCaspase11-/- (Casp1-/-Casp11-/-) and Nlrp3-/- BMDMs stimulated with EC for 1.5h. Arrows indicate the main SCs affected. (n) Schematics of the consequences of bacteria detection on macrophage mitochondrial respiratory chain and metabolism. NS, not significant; *P < 0.05; ***P < 0.001 (two-tailed unpaired Student’s t-test or one-way ANOVA followed by Tukey post-test analysis (a)). Data are from three (a, d, i, k), four (e, f) and five (g) independent experiments performed in duplicates. Data in (c) present mean and s.d. of one representative experiment performed in six technical replicates. Data in (b, l, j, m) are from one representative of three independent experiments with similar results.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1422 kb)
Rights and permissions
About this article
Cite this article
Garaude, J., Acín-Pérez, R., Martínez-Cano, S. et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat Immunol 17, 1037–1045 (2016). https://doi.org/10.1038/ni.3509
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3509
This article is cited by
-
Mitochondrial translocation of TFEB regulates complex I and inflammation
EMBO Reports (2024)
-
Inflammatory macrophages reprogram to immunosuppression by reducing mitochondrial translation
Nature Communications (2023)
-
Trained immunity — basic concepts and contributions to immunopathology
Nature Reviews Nephrology (2023)
-
A FRET-based respirasome assembly screen identifies spleen tyrosine kinase as a target to improve muscle mitochondrial respiration and exercise performance in mice
Nature Communications (2023)
-
Metabolism of tissue macrophages in homeostasis and pathology
Cellular & Molecular Immunology (2022)