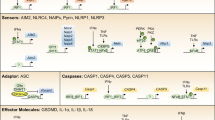
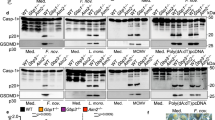
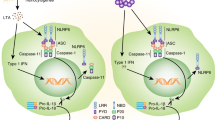
Abstract
Traditional views of the inflammasome highlight the assembly of pre-existing core components shortly after infection or tissue damage. Emerging work, however, suggests that the inflammasome machinery is also subject to 'tunable' or inducible signals that might accelerate its autocatalytic properties and dictate where inflammasome assembly takes place in the cell. Many of these signals operate downstream of interferon receptors to elicit inflammasome regulators, including a new family of interferon-induced GTPases called 'guanylate-binding proteins' (GBPs). Here we investigate the critical roles of interferon-induced GBPs in directing inflammasome subtype–specific responses and their consequences for cell-autonomous immunity to a wide variety of microbial pathogens. We discuss emerging mechanisms of action and the potential effect of these GBPs on predisposition to sepsis and other infectious or inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Daugherty, M.D. & Malik, H.S. Rules of engagement: molecular insights from host-virus arms races. Annu. Rev. Genet. 46, 677–700 (2012).
Aravind, L., Dixit, V.M. & Koonin, E.V. Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science 291, 1279–1284 (2001).
MacMicking, J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12, 367–382 (2012).
Lamkanfi, M. & Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Angosto, D. et al. Evolution of inflammasome functions in vertebrates: Inflammasome and caspase-1 trigger fish macrophage cell death but are dispensable for the processing of IL-1β. Innate Immun. 18, 815–824 (2012).
Henry, T., Brotcke, A., Weiss, D.S., Thompson, L.J. & Monack, D.M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Jones, J.W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107, 9771–9776 (2010).
Rathinam, V.A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Dombrowski, Y. et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci. Transl. Med. 3, 82ra38 (2011).
Kerur, N. et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 (2011).
Rathinam, V.A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Shenoy, A.R. et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 (2012).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Case, C.L. et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl. Acad. Sci. USA 110, 1851–1856 (2013).
Hagar, J.A., Powell, D.A., Aachoui, Y., Ernst, R.K. & Miao, E.A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Strittmatter, G.E. et al. IFN-γ primes keratinocytes for HSV-1-induced inflammasome activation. J. Invest. Dermatol. 136, 610–620 (2016).
Pilla, D.M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl. Acad. Sci. USA 111, 6046–6051 (2014).
Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014).
Aachoui, Y. et al. Canonical inflammasomes drive IFN-γ to prime caspase-11 in defense against a cytosol-invasive bacterium. Cell Host Microbe 18, 320–332 (2015).
Finethy, R. et al. Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in chlamydia-infected macrophages. Infect. Immun. 83, 4740–4749 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Man, S.M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015).
Meunier, E. et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484 (2015).
Kim, B.H., Shenoy, A.R., Kumar, P., Bradfield, C.J. & MacMicking, J.D. IFN-inducible GTPases in host cell defense. Cell Host Microbe 12, 432–444 (2012).
Latz, E., Xiao, T.S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
von Moltke, J., Ayres, J.S., Kofoed, E.M., Chavarría-Smith, J. & Vance, R.E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31, 73–106 (2013).
Chen, K.W. et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8, 570–582 (2014).
Knodler, L.A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Casson, C.N. et al. Human caspase-4 mediates noncanonical inflammasome activation against Gram-negative bacterial pathogens. Proc. Natl. Acad. Sci. USA 112, 6688–6693 (2015).
Broz, P., von Moltke, J., Jones, J.W., Vance, R.E. & Monack, D.M. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483 (2010).
Broz, P. et al. Caspase-11 increases susceptibility to salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222 (2014).
Hu, Z. et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science 350, 399–404 (2015).
Zhang, L. et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Franchi, L., Eigenbrod, T. & Núñez, G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 183, 792–796 (2009).
Lee, C.K., Smith, E., Gimeno, R., Gertner, R. & Levy, D.E. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-γ. J. Immunol. 164, 1286–1292 (2000).
Schauvliege, R., Vanrobaeys, J., Schotte, P. & Beyaert, R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-κB and signal transducer and activator of transcription (STAT) 1. J. Biol. Chem. 277, 41624–41630 (2002).
Lu, B. et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 (2012).
He, Y., Franchi, L. & Núñez, G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur. J. Immunol. 43, 1147–1152 (2013).
Hett, E.C. et al. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat. Chem. Biol. 9, 398–405 (2013).
Kim, B.H. et al. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332, 717–721 (2011).
Jin, T., Huang, M., Smith, P., Jiang, J. & Xiao, T.S. Structure of the caspase-recruitment domain from a zebrafish guanylate-binding protein. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69, 855–860 (2013).
Olszewski, M.A., Gray, J. & Vestal, D.J. In silico genomic analysis of the human and murine guanylate-binding protein (GBP) gene clusters. J. Interferon Cytokine Res. 26, 328–352 (2006).
Degrandi, D. et al. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J. Immunol. 179, 7729–7740 (2007).
Shenoy, A.R. et al. Emerging themes in IFN-γ-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiology 212, 771–784 (2007).
MacMicking, J.D., Taylor, G.A. & McKinney, J.D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302, 654–659 (2003).
Martens, S. et al. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 1, e24 (2005).
Staeheli, P., Prochazka, M., Steigmeier, P.A. & Haller, O. Genetic control of interferon action: mouse strain distribution and inheritance of an induced protein with guanylate-binding property. Virology 137, 135–142 (1984).
Kim, S. et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur. J. Immunol. 40, 1545–1551 (2010).
Rupper, A.C. & Cardelli, J.A. Induction of guanylate binding protein 5 by γ interferon increases susceptibility to Salmonella enterica serovar Typhimurium-induced pyroptosis in RAW 264.7 cells. Infect. Immun. 76, 2304–2315 (2008).
Marty-Roix, R. et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 291, 1123–1136 (2016).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Sheedy, F.J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Okada, M., Matsuzawa, A., Yoshimura, A. & Ichijo, H. The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J. Biol. Chem. 289, 32926–32936 (2014).
Randow, F., MacMicking, J.D. & James, L.C. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 340, 701–706 (2013).
Pierini, R. et al. ASC controls IFN-γ levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida. J. Immunol. 191, 3847–3857 (2013).
Yamamoto, M. et al. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 37, 302–313 (2012).
Wehner, M. & Herrmann, C. Biochemical properties of the human guanylate binding protein 5 and a tumor-specific truncated splice variant. FEBS J. 277, 1597–1605 (2010).
Ghosh, A., Praefcke, G.J., Renault, L., Wittinghofer, A. & Herrmann, C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature 440, 101–104 (2006).
Syguda, A. et al. Tetramerization of human guanylate-binding protein 1 is mediated by coiled-coil formation of the C-terminal α-helices. FEBS J. 279, 2544–2554 (2012).
Abdullah, N., Srinivasan, B., Modiano, N., Cresswell, P. & Sau, A.K. Role of individual domains and identification of internal gap in human guanylate binding protein-1. J. Mol. Biol. 386, 690–703 (2009).
Britzen-Laurent, N. et al. Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner. PLoS One 5, e14246 (2010).
Degrandi, D. et al. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc. Natl. Acad. Sci. USA 110, 294–299 (2013).
Selleck, E.M. et al. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 9, e1003320 (2013).
Tietzel, I., El-Haibi, C. & Carabeo, R.A. Human guanylate binding proteins potentiate the anti-chlamydia effects of interferon-γ. PLoS One 4, e6499 (2009).
Virreira Winter, S. et al. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One 6, e24434 (2011).
Lee, Y. et al. p62 plays a specific role in interferon-γ-induced presentation of a toxoplasma vacuolar antigen. Cell Rep. 13, 223–233 (2015).
Niedelman, W., Sprokholt, J.K., Clough, B., Frickel, E.M. & Saeij, J.P. Cell death of γ interferon-stimulated human fibroblasts upon Toxoplasma gondii infection induces early parasite egress and limits parasite replication. Infect. Immun. 81, 4341–4349 (2013).
Ohshima, J. et al. Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J. Immunol. 192, 3328–3335 (2014).
Selleck, E.M. et al. A noncanonical autophagy pathway restricts Toxoplasma gondii growth in a strain-specific manner in IFN-γ-activated human cells. MBio 6, e01157–e15 (2015).
Kagan, J.C., Magupalli, V.G. & Wu, H. SMOCs: supramolecular organizing centres that control innate immunity. Nat. Rev. Immunol. 14, 821–826 (2014).
Kravets, E. et al. The GTPase activity of murine guanylate-binding protein 2 (mGBP2) controls the intracellular localization and recruitment to the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 287, 27452–27466 (2012).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
MacMicking, J.D. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol. 25, 601–609 (2004).
Tiwari, S., Choi, H.P., Matsuzawa, T., Pypaert, M. & MacMicking, J.D. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P2 and PtdIns(3,4,5)P3 promotes immunity to mycobacteria. Nat. Immunol. 10, 907–917 (2009).
Haldar, A.K. et al. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 9, e1003414 (2013).
Ohtsuka, S. et al. SQSTM1/p62/A170 regulates the severity of Legionella pneumophila pneumonia by modulating inflammasome activity. Eur. J. Immunol. 44, 1084–1092 (2014).
Haldar, A.K. et al. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc. Natl. Acad. Sci. USA 112, E5628–E5637 (2015).
Hotchkiss, R.S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013).
MacMicking, J.D. et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 (1995).
Döcke, W.D. et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat. Med. 3, 678–681 (1997).
Broderick, L., De Nardo, D., Franklin, B.S., Hoffman, H.M. & Latz, E. The inflammasomes and autoinflammatory syndromes. Annu. Rev. Pathol. 10, 395–424 (2015).
Crow, Y.J. & Manel, N. Aicardi-Goutières syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Jeremiah, N. et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 124, 5516–5520 (2014).
Acknowledgements
Supported by the HHMI Investigator Program, NIH NIAID (R01 AI068041-10 and R01 AI108834-02), the American Asthma Foundation Research Program (14-0073) and the Rainin Foundation (14H7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kim, BH., Chee, J., Bradfield, C. et al. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat Immunol 17, 481–489 (2016). https://doi.org/10.1038/ni.3440
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3440
This article is cited by
-
A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis
Cell Research (2023)
-
Dnmt1/Tet2-mediated changes in Cmip methylation regulate the development of nonalcoholic fatty liver disease by controlling the Gbp2-Pparγ-CD36 axis
Experimental & Molecular Medicine (2023)
-
Subversion of GBP-mediated host defense by E3 ligases acquired during Yersinia pestis evolution
Nature Communications (2022)
-
Predictive urinary RNA biomarkers of kidney injury after extracorporeal shock wave lithotripsy
World Journal of Urology (2022)
-
When human guanylate-binding proteins meet viral infections
Journal of Biomedical Science (2021)