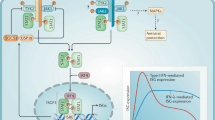
Abstract
Mucosal surfaces are exposed to environmental substances and represent a major portal of entry for microorganisms. The innate immune system is responsible for early defense against infections and it is believed that the interferons (IFNs) constitute the first line of defense against viruses. Here we identify an innate antiviral pathway that works at epithelial surfaces before the IFNs. The pathway is activated independently of known innate sensors of viral infections through a mechanism dependent on viral O-linked glycans, which induce CXCR3 chemokines and stimulate antiviral activity in a manner dependent on neutrophils. This study therefore identifies a previously unknown layer of antiviral defense that exerts its action on epithelial surfaces before the classical IFN response is operative.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Iwasaki, A. Antiviral immune responses in the genital tract: clues for vaccines. Nat. Rev. Immunol. 10, 699–711 (2010).
Ichinohe, T., Iwasaki, A. & Hasegawa, H. Innate sensors of influenza virus: clues to developing better intranasal vaccines. Expert Rev. Vaccines 7, 1435–1445 (2008).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Janeway, C.A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Okabe, Y., Kawane, K., Akira, S., Taniguchi, T. & Nagata, S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 202, 1333–1339 (2005).
Imai, Y. et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 (2008).
Gupta, R., Warren, T. & Wald, A. Genital herpes. Lancet 370, 2127–2137 (2007).
Kurt-Jones, E.A. et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101, 1315–1320 (2004).
Sørensen, L.N. et al. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 181, 8604–8612 (2008).
Reinert, L.S. et al. TLR3-deficiency renders astrocytes permissive to HSV infection and facilitates establishment of CNS infection in mice. J. Clin. Invest. 122, 1368–1376 (2012).
Melchjorsen, J. et al. Innate recognition of HSV in human primary macrophages is mediated via the MDA5/MAVS pathway and MDA5/MAVS/Pol III independent pathways. J. Virol. 84, 11350–11358 (2010).
Ishikawa, H., Ma, Z. & Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Li, X.D. et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Paludan, S.R., Bowie, A.G., Horan, K.A. & Fitzgerald, K.A. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11, 143–154 (2011).
Crotta, S. et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 9, e1003773 (2013).
Leib, D.A. et al. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189, 663–672 (1999).
Mordstein, M. et al. Interferon-λ renders epithelial cells of respiratory and gastrointestinal tract resistant to viral infections. J. Virol. 84, 5670–5677 (2010).
Zhao, X. et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective TH1 responses to herpes simplex virus-2. J. Exp. Med. 197, 153–162 (2003).
Groom, J.R. & Luster, A.D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89, 207–215 (2011).
Cheshenko, N. et al. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J. 27, 2584–2599 (2013).
Radhakrishnan, P. et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 111, E4066–E4075 (2014).
Bagdonaite, I. et al. A strategy for O-glycoproteomics of enveloped viruses–the O-glycoproteome of herpes simplex virus type 1. PLoS Pathog. 11, e1004784 (2015).
McGuckin, M.A., Linden, S.K., Sutton, P. & Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011).
Harrington, S.M. et al. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 77, 2465–2473 (2009).
Frazer, L.C., O'Connell, C.M., Andrews, C.W. Jr., Zurenski, M.A. & Darville, T. Enhanced neutrophil longevity and recruitment contribute to the severity of oviduct pathology during Chlamydia muridarum infection. Infect. Immun. 79, 4029–4041 (2011).
Archer, N.K., Harro, J.M. & Shirtliff, M.E. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect. Immun. 81, 2070–2075 (2013).
Milligan, G.N. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73, 6380–6386 (1999).
Jenne, C.N. et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 13, 169–180 (2013).
Ohmori, Y., Schreiber, R.D. & Hamilton, T.A. Synergy between interferon-γ and tumor necrosis factor-α in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor-κB. J. Biol. Chem. 272, 14899–14907 (1997).
Ohmori, Y. & Hamilton, T.A. The interferon-stimulated response element and a κB site mediate synergistic induction of murine IP-10 gene transcription by IFN-γ and TNF-α. J. Immunol. 154, 5235–5244 (1995).
Hollingsworth, M.A. & Swanson, B.J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60 (2004).
Dodds, P.N. & Rathjen, J.P. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548 (2010).
Pinheiro, J.C. & Bates, D.M. in Mixed Effects Models in S and S-PLUS 273–301 (Springer, 2000).
Acknowledgements
We thank K.S. Petersen and I.M. Poulsen for technical assistance and the AU FACS Core facility for technical help. Supported by The Danish Medical Research Council (12-124330 to S.R.P.; 1331-00133B to H.H.W.), the Novo Nordisk Foundation (S.R.P.), the Lundbeck Foundation (R34-3855 to S.R.P.), Aarhus University Research Foundation (S.R.P.), the EU FP7 Mobilex program (1333-00090A to M.K.T.), the US National Institutes of Health (AI065309 to B.C.H.), Faculty of Health Sciences, AU (M.B.I.), the Danish National Research Foundation (DNRF107 to H.H.W.), the Excellence Programme for Interdisciplinary Research (CDO2016 to H.H.W.), the German Research Foundation (DFG, SFB 1054 to J.R.) and the European Research Council (FP7, grant agreement 322865 to J.R.).
Author information
Authors and Affiliations
Contributions
M.B.I., C.K.H. and S.R.P. conceived of the study; M.B.I., L.S.R., M.K.T., I.B., R.N., N.C., T.P., S.Y.V., M.K. and S.K.K. performed experiments; M.B.I., B.M.B., H.H.W., S.F., C.K.H. and S.R.P. analyzed data; F.R.-P., S.G., K.E., J.R., A.R.T., B.C.H., S.V.P. and H.H.W. provided reagents and intellectual guidance; and M.B.I. and S.R.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
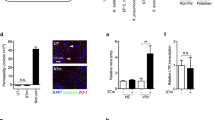
Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection.
C57BL/6 mice were (a) treated intravaginally with 20 µL of PBS or infected with 6.7x104 pfu of HSV-2 in the same volume, or were (b) infected intravaginally with 6.7x104 pfu of HSV-2 produced in murine neurons. Vaginal washes were isolated 24 h p.i. and levels of CXCL10 were determined by ELISA. (c-k) C57BL/6 mice were infected intravaginally with 6.7x104 pfu of HSV-2. Vaginal washes were isolated at the indicated time points p.i., and analyzed for levels of (c) CCL2, (d) CCL5, (e) CXCL1, (f) IL-1β, (g) IL-10, (h) IL-12 p40, (i) IL-15, (j) IL-33, (k) TNF-α. Data are presented as means +/- st.dev. n = 6-8 per group. ** 0.001<p<0.01; *** p<0.001; ns, not statistically significant.
Supplementary Figure 2 An essential role for CXCR3 in early control of corneal HSV-1 infection.
C57BL/6 and Cxcr3-/- mice were infected in the cornea with 2x106 pfu of HSV-1 (McKrae). (a). Eye balls were isolated 24 h p.i. and analyzed for the levels of CXCL10 and bioactive type I IFN. n=8 per group. (b) Total RNA isolated from homogenized eyes of mice infected for 24 h was analyzed for gB and Gapdh mRNA by RT-qPCR. Data are presented as means +/- standard deviation n=5-8 per group. * 0.01<p<0.05, ** 0.001<p<0.01, *** p<0.001.
Supplementary Figure 3 Type II and III IFNs are redundant for innate control of genital HSV-2 infection.
C57BL/6, Il28ra-/-, and Ifngr-/- mice were infected intravaginally with 6.7x104 pfu of HSV-2. (a, c) Vaginal washes were isolated on day 1 and 2 points p.i., and analyzed for viral load by plaque assay on Vero cells. Data points represent individual mice (n= 5-10 per group). (b, d) The mice were scored daily for clinical signs of disease. Data are presented as mean disease score +/- standard deviation. ns, not statistically significant.
Supplementary Figure 4 Re-establishment of a mucosal layer by vaginal epithelial cells in vitro.
Vaginal tissue was excised from female mice and treated with Collagenase and Dispase as described in the method section. The subepithelium was removed, and the epithelial cell layer was sat in culture for 24 h. (a) Total RNA was isolated and analyzed for expression of Muc1, 2, 5b, and Gapdh. For comparison, RNA from murine bone-marrow-derived dendritic cells (BMDCs) was analyzed in parallel. Data are presented as means +/- st.dev. n=3 per group. (b) Periodic acid–Schiff staining of vaginal epithelial cells in culture after the treatment described above. As control is included a staining of a section from vaginal tissue treated with medium not supplemented with Collagenase/Dispase. The periodic acid–Schiff stains glycans, which are abundantly present in the mucus layer (red).
Supplementary Figure 5 Expression of PRRs in the vagina of mice.
(a) RNA was isolated from vaginas and bone-marrow-derived dendritic cells from C57BL/6 mice. Levels of Tlr2, Tlr3, Tlr9, Rig-I, Mda5, Mb21d1 (cGAS) and β-actin mRNA were measured by RT-qPCR. For each PRR, data was normalized to β-actin and expression levels are presented as mean of the ratio between vaginal epithelial cells and dendritic cells +/- standard deviation. n = 3. (b) The Ct values from the PCR data set on dendritic cells used for calculation of expression ratios.
Supplementary Figure 6 Development of disease after infection with infectious or attenuated HSV-2.
C57BL/6 mice were infected intravaginally with 6.7x104 pfu of infectious or UV-inactivated HSV-2 or HSV-2 ΔgD-R (revertant) or HSV-2 ΔgD. (a) On subsequent days, the animals were scored for clinical signs of disease (n = 8). Data are presented as mean disease score +/- st. dev. (b) Tissue sections of the vagina from mice left untreated or infected for 24 h with HSV-2 or HSV-2 ΔgD were stained with anti-HSV-2. Scale bar, 100 μm.
Supplementary Figure 7 Characterization of HSV-2 devoid of O-linked sugars.
(a-d) HSV-2 stocks were treated as indicated for 30 min at 37oC and subjected to (a) SDS-PAGE and Western blotting using antibodies against gB and gD, (b, d) infection on MEFs followed by confocal microscopy (stainings: anti-VP5, anti-STING, and DAPI) and (c) viral plaque titration on Vero cells. (e) EdC-labeled HSV-2 grown in parental or Cosmc KO cells were incubated at 37 °C for 30 min. DNA and capsid were visualized with CLICK chemistry and anti-VP5 respectively. Data are presented as mean % CLICK+VP5- foci of the total number of counted VP5+ foci +/- st.dev. (f-g) C57BL/6 mice were infected intravaginally with 6.7x104 pfu of (f) HSV-2, which has been treated with buffer or an enzyme mix cleaving O-linked sugars before infection, or (g) HSV-2 grown in either parental or Cosmc KO HaCaT cells. Vaginal washes were harvested 48 hours p.i. for and levels of CXCL10 were measured (n= 8). (h) Virus titer for preparations of HSV-2 grown in parental or Cosmc KO HaCaT cells. Data are presented as means +/- st.dev. * 0.01<p<0.05, ** 0.001<p<0.01, *** p<0.001. ns, not statistically significant.
Supplementary Figure 8 Identification of NK cells and neutrophils by flow cytometry.
C57BL/6 mice were infected intravaginally with 6.7x104 pfu of HSV-2 or left untreated. 24 hours p.i., single-cell leukocytes were isolated from the vaginal tissue and analyzed by flow cytometry (a) Ly6G+CD11b+ cells, and (b) CD45+ NK1.1+ cells. The plots represent one infected C57BL/6 mouse and one naive C57BL/6 mouse. (c) Vaginal washes were isolated 24 hours p.i. and analyzed for levels of CXCL10. Data are presented as means +/- standard deviation. n = 8 per group. ns, not statistically significant.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 2 (PDF 1784 kb)
Supplementary Table 1
Supplementary information on mass spectrometry analysis of HSV-2 O-glycoproteome (XLSX 122 kb)
Rights and permissions
About this article
Cite this article
Iversen, M., Reinert, L., Thomsen, M. et al. An innate antiviral pathway acting before interferons at epithelial surfaces. Nat Immunol 17, 150–158 (2016). https://doi.org/10.1038/ni.3319
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3319
This article is cited by
-
Glycoengineered keratinocyte library reveals essential functions of specific glycans for all stages of HSV-1 infection
Nature Communications (2023)
-
TRPV4 channel is involved in HSV-2 infection in human vaginal epithelial cells through triggering Ca2+ oscillation
Acta Pharmacologica Sinica (2023)
-
Modeling human HSV infection via a vascularized immune-competent skin-on-chip platform
Nature Communications (2022)
-
Neutrophils in cancer carcinogenesis and metastasis
Journal of Hematology & Oncology (2021)
-
Constitutive immune mechanisms: mediators of host defence and immune regulation
Nature Reviews Immunology (2021)