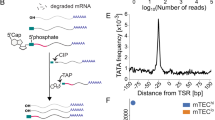
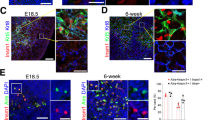
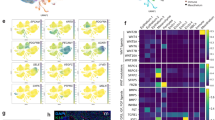
Abstract
Expression of tissue-restricted self antigens (TRAs) in medullary thymic epithelial cells (mTECs) is essential for the induction of self-tolerance and prevents autoimmunity, with each TRA being expressed in only a few mTECs. How this process is regulated in single mTECs and is coordinated at the population level, such that the varied single-cell patterns add up to faithfully represent TRAs, is poorly understood. Here we used single-cell RNA sequencing and obtained evidence of numerous recurring TRA–co-expression patterns, each present in only a subset of mTECs. Co-expressed genes clustered in the genome and showed enhanced chromatin accessibility. Our findings characterize TRA expression in mTECs as a coordinated process that might involve local remodeling of chromatin and thus ensures a comprehensive representation of the immunological self.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Anderson, M.S. et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002).
DeVoss, J.J. & Anderson, M.S. Lessons on immune tolerance from the monogenic disease APS1. Curr. Opin. Genet. Dev. 17, 193–200 (2007).
Hogquist, K.A., Baldwin, T.A. & Jameson, S.C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782 (2005).
Klein, L., Kyewski, B., Allen, P.M. & Hogquist, K.A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don′t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2, 1032–1039 (2001).
Kyewski, B. & Klein, L. A central role for central tolerance. Annu. Rev. Immunol. 24, 571–606 (2006).
Perry, J.S. et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41, 414–426 (2014).
Yang, S., Fujikado, N., Kolodin, D., Benoist, C. & Mathis, D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594 (2015).
Malchow, S. et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224 (2013).
Derbinski, J., Pinto, S., Rosch, S., Hexel, K. & Kyewski, B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc. Natl. Acad. Sci. USA 105, 657–662 (2008).
Cloosen, S. et al. Expression of tumor-associated differentiation antigens, MUC1 glycoforms and CEA, in human thymic epithelial cells: implications for self-tolerance and tumor therapy. Cancer Res. 67, 3919–3926 (2007).
Pinto, S. et al. Overlapping gene coexpression patterns in human medullary thymic epithelial cells generate self-antigen diversity. Proc. Natl. Acad. Sci. USA 110, E3497–E3505 (2013).
Mathis, D. & Benoist, C. Aire. Annu. Rev. Immunol. 27, 287–312 (2009).
Abramson, J., Giraud, M., Benoist, C. & Mathis, D. Aire's partners in the molecular control of immunological tolerance. Cell 140, 123–135 (2010).
Derbinski, J. et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202, 33–45 (2005).
Koh, A.S. et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc. Natl. Acad. Sci. USA 105, 15878–15883 (2008).
Org, T. et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 9, 370–376 (2008).
Waterfield, M. et al. The transcriptional regulator Aire coopts the repressive ATF7ip-MBD1 complex for the induction of immunotolerance. Nat. Immunol. 15, 258–265 (2014).
Sansom, S.N. et al. Population and single cell genomics reveal the Aire-dependency, relief from Polycomb silencing and distribution of self-antigen expression in thymic epithelia. Genome Res. 24, 1918–1931 (2014).
Giraud, M. et al. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc. Natl. Acad. Sci. USA 109, 535–540 (2012).
Villaseñor, J., Besse, W., Benoist, C. & Mathis, D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc. Natl. Acad. Sci. USA 105, 15854–15859 (2008).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
St-Pierre, C., Brochu, S., Vanegas, J.R., Dumont-Lagace, M., Lemieux, S. & Perreault, C. Transcriptome sequencing of neonatal thymic epithelial cells. Sci. Rep. 3, 1860 (2013).
Brennecke, P. et al. Accounting for technical noise in single-cell RNA-seq experiments. Nat. Methods 10, 1093–1095 (2013).
Forrest, A.R. et al. A promoter-level mammalian expression atlas. Nature 507, 462–470 (2014).
Buettner, F. et al. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat. Biotechnol. 33, 155–160 (2015).
Ohnishi, Y. et al. Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat. Cell Biol. 16, 27–37 (2014).
Gotter, J., Brors, B., Hergenhahn, M. & Kyewski, B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J. Exp. Med. 199, 155–166 (2004).
Johnnidis, J.B. et al. Chromosomal clustering of genes controlled by the aire transcription factor. Proc. Natl. Acad. Sci. USA 102, 7233–7238 (2005).
Buenrostro, J.D., Giresi, P.G., Zaba, L.C., Chang, H.Y. & Greenleaf, W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Ramsköld, D. et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Tang, F. et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat. Protoc. 5, 516–535 (2010).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Islam, S. et al. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res. 21, 1160–1167 (2011).
Islam, S. et al. Highly multiplexed and strand-specific single-cell RNA 5′ end sequencing. Nat. Protoc. 7, 813–828 (2012).
Le Borgne, M. et al. The impact of negative selection on thymocyte migration in the medulla. Nat. Immunol. 10, 823–830 (2009).
Pinto, S. et al. Misinitiation of intrathymic MART-1 transcription and biased TCR usage explain the high frequency of MART-1-specific T cells. Eur. J. Immunol. 44, 2811–2821 (2014).
Klein, L., Klugmann, M., Nave, K.A., Tuohy, V.K. & Kyewski, B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat. Med. 6, 56–61 (2000).
Schoenfelder, S. et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat. Genet. 42, 53–61 (2010).
Tykocinski, L.O. et al. Epigenetic regulation of promiscuous gene expression in thymic medullary epithelial cells. Proc. Natl. Acad. Sci. USA 107, 19426–19431 (2010).
Azuara, V. et al. Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 8, 532–538 (2006).
Bert, S.A. et al. Regional activation of the cancer genome by long-range epigenetic remodeling. Cancer Cell 23, 9–22 (2013).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Rattay, K. et al. Homeodomain-interacting protein kinase 2, a novel autoimmune regulator interaction partner, modulates promiscuous gene expression in medullary thymic epithelial cells. J. Immunol. 194, 921–928 (2015).
Farr, A., Nelson, A., Truex, J. & Hosier, S. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J. Histochem. Cytochem. 39, 645–653 (1991).
Rouse, R.V., Bolin, L.M., Bender, J.R. & Kyewski, B.A. Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J. Histochem. Cytochem. 36, 1511–1517 (1988).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Acknowledgements
We thank K. Hexel and S. Schmitt for single-cell sorting; S. Egle for technical help; C. Sebening and T. Loukanov (University of Heidelberg) for human thymic tissue; The Genomics Core Facility of the European Molecular Biology Laboratory for initial sequencing, and M. Miranda and E. Hopmans for support during subsequent sequencing at the Stanford Genome Technology Center; J. Buenrostro and C. Chabbert for discussions about ATAC-seq experiments and data, respectively; C. Michel and S. Anders for advice and comments on the manuscript; W. Wei and M. Sikora for help with data transfer; and The Central Animal Facility (German Cancer Research Center) for animal care. Supported by the European Union 7th Framework Programme (Health) via Project Radiant (W.H. and A.R.), The Helmholtz Center (K.R.), the Sonderforschungsbereich (DFG 938 to S.P.), the European Research Council (ERC-2012-AdG to B.K.) and the US National Institutes of Health (P01 HG000205 and R01 GM068717 to P.B., M.N. and L.M.S.).
Author information
Authors and Affiliations
Contributions
P.B., S.P., B.K. and L.M.S. conceived of the project; P.B., S.P. and K.R. designed experiments; P.B. performed single-cell sequencing experiments, Klk5 single-cell quantitative PCR confirmation experiments and ATAC-seq experiments; S.P. helped with the ATAC-seq experiments; S.P. and K.R. performed experimental mTEC preparations and flow cytometry of single and bulk mTECs; A.R. and W.H. designed analysis strategy and analyzed the data; A.R. prepared the figures; P.B., A.R., S.P., K.R., W.H., B.K. and L.M.S. interpreted the data and wrote the manuscript; M.N. and R.K. provided technical assistance; and L.M.S., B.K. and W.H. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Histogram of the fraction of genes detected per mTEC that are classified as TRA-encoding genes.
The x-axis shows the fraction of genes detected per cell that are classified as TRA-encoding genes. The y-axis shows the number of cells.
Supplementary Figure 2 Enrichment of TRA-encoding genes among highly variable genes.
Barplot showing the fraction of TRA-encoding genes (y-axis) in two subsets of genes (x-axis), those that were detected to be highly variable in Fig 1c and the rest of the genes that were not detected to be highly variable (See Methods for details). The difference in the fraction of genes overlapping with TRA-encoding genes was detected to be significantly different between the two gene subsets (p-value < 2.2 x 10-16, Fisher’s exact test).
Supplementary Figure 3 Enrichment of genes in the Tspan8–co-expressed gene set among genes from cluster B resulting from the k-medoids clustering.
Barplot showing the fraction of genes from the Tspan8 co-expressed gene set (y-axis) in two subsets of genes (x-axis), genes belonging to cluster B resulting from the k-medoids clustering (Fig. 2a) and genes grouped to the rest of the k-medoids clusters (See Methods for details). The difference in the fraction of genes overlapping with the Tspan8 co-expressed gene set was detected to be significantly different between the two gene subsets (p-value < 2.2 x 10-16, Fisher’s exact test).
Supplementary Figure 4 Confirmation of co-expressed gene sets by independent experimental approaches.
Each point depicts one gene. The y-axis shows the logarithmic fold change (base 2) of the unselected mature mTECs where TRA expression was detected by the scRNA-seq assay with respect to the unselected mature mTECs where the scRNA-seq assay did not detect the expression of the TRA. The x-axis shows the logarithmic fold change (base 2) between the mature mTECs selected for the expression of the TRA (by either qPCR or flow cytometry) and the unselected mature mTECs where the scRNA-seq assay did not detect the expression of the TRA. Each column panel shows the data for one TRA. The row panels split the genes according to whether they were detected to be co-expressed with the specific TRA or not.
Supplementary Figure 5 Enrichment of genes in the Klk5–co-expressed gene set among genes from cluster D resulting from the k-medoids clustering.
Barplot showing the fraction of genes from the Klk5 co-expressed gene set (y-axis) in two subsets of genes (x-axis), genes belonging to cluster D resulting from the k-medoids clustering and genes grouped to the rest of the k-medoids clusters (See Methods for details). The difference in the fraction of genes overlapping with the Klk5 co-expressed gene set was detected to be significantly different between the two gene subsets (p-value < 9.6 x 10-4, Fisher’s exact test).
Supplementary Figure 6 Expected versus observed genomic proximity of the 11 groups of co-expressed genes resulting from the k-medoids clustering.
Each panel shows the data for one of the 11 clusters from Fig. 2b. The histograms show the expected distribution of the median genomic distance between pairs of genes in the genome according to the size of each co-expressed gene set. This expected distribution was estimated by sampling random genes of the same size of the co-expressed gene set for 1,000 times. The observed median distance for each cluster is depicted with vertical solid lines.
Supplementary Figure 7 Karyogram of the genomic position of the 11 groups of co-expressed genes resulting from the k-medoids clustering.
Each panel corresponds to one gene cluster. The colors correspond to the colors from Fig. 2. The colored vertical lines in the chromosomes mark the genomic position of genes.
Supplementary Figure 8 Examples of co-expressed genomic loci and their locations in the genome.
(a) The genomic locus of the BPI fold-containing B gene family is shown. The x-axis represents the genomic positions from chromosome 2 of the mouse genome. Protein coding genes are shown in boxes. Genes colored in purple belong to cluster “D”. (+) = plus strand, (-) = minus strand. (b) The genomic locus of the Glutathione S-transferase Mu gene family is shown. The x-axis represents the genomic positions from chromosome 3 of the mouse genome. Protein coding genes are shown in boxes. Genes colored in purple belong to cluster “D”. (+) = plus strand, (-) = minus strand. (c) Example of co-expressed genes from unrelated gene families that are clustered in the genome. The x-axis represents the genomic positions from chromosome 6 of the mouse genome. Protein coding genes are shown in boxes. Genes colored in purple belong to cluster “D”. (+) = plus strand, (-) = minus strand.
Supplementary Figure 9 Fraction of cells with detection of expression of genes encoding proteases of the kallikrein family.
The y-axis shows the fraction of cells for which the scRNA-seq assay detected gene expression. The x-axis shows genes ordered by genomic location. The upper panel shows the data for the qPCR-selected Klk5+ cells. The lower panel shows the data for the unselected mature mTECs for which Klk5 expression was not detected by the scRNA-seq assay.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 964 kb)
Supplementary Code 1
Single-cell transcriptome analysis reveals coordinated ectopic gene expression patterns in medullary thymic epithelial cells (PDF 15619 kb)
Rights and permissions
About this article
Cite this article
Brennecke, P., Reyes, A., Pinto, S. et al. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat Immunol 16, 933–941 (2015). https://doi.org/10.1038/ni.3246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3246
This article is cited by
-
AIRE relies on Z-DNA to flag gene targets for thymic T cell tolerization
Nature (2024)
-
Thymic self-antigen expression for immune tolerance and surveillance
Inflammation and Regeneration (2022)
-
Indispensable epigenetic control of thymic epithelial cell development and function by polycomb repressive complex 2
Nature Communications (2021)
-
Leaving no one behind: tracing every human thymocyte by single-cell RNA-sequencing
Seminars in Immunopathology (2021)
-
Thymic epithelial cell heterogeneity: TEC by TEC
Nature Reviews Immunology (2020)