Abstract
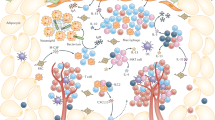
Fat-associated lymphoid clusters (FALCs) are a type of lymphoid tissue associated with visceral fat. Here we found that the distribution of FALCs was heterogeneous, with the pericardium containing large numbers of these clusters. FALCs contributed to the retention of B-1 cells in the peritoneal cavity through high expression of the chemokine CXCL13, and they supported B cell proliferation and germinal center differentiation during peritoneal immunological challenges. FALC formation was induced by inflammation, which triggered the recruitment of myeloid cells that expressed tumor-necrosis factor (TNF) necessary for signaling via the TNF receptors in stromal cells. Natural killer T cells (NKT cells) restricted by the antigen-presenting molecule CD1d were likewise required for the inducible formation of FALCs. Thus, FALCs supported and coordinated the activation of innate B cells and T cells during serosal immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Silverman, G.J., Gronwall, C., Vas, J. & Chen, Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov. Med. 8, 151–156 (2009).
Alugupalli, K.R. et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390 (2004).
Ochsenbein, A.F. et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286, 2156–2159 (1999).
Baumgarth, N. et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192, 271–280 (2000).
Haas, K.M., Poe, J.C., Steeber, D.A. & Tedder, T.F. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23, 7–18 (2005).
Boes, M., Prodeus, A.P., Schmidt, T., Carroll, M.C. & Chen, J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188, 2381–2386 (1998).
Martin, F., Oliver, A.M. & Kearney, J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14, 617–629 (2001).
Platell, C., Cooper, D., Papadimitriou, J.M. & Hall, J.C. The omentum. World J. Gastroenterol. 6, 169–176 (2000).
Ansel, K.M., Harris, R.B. & Cyster, J.G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16, 67–76 (2002).
Carlow, D.A., Gold, M.R. & Ziltener, H.J. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J. Immunol. 183, 1155–1165 (2009).
Ha, S.A. et al. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203, 2541–2550 (2006).
Rangel-Moreno, J. et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 30, 731–743 (2009).
Elewa, Y.H., Ichii, O., Otsuka, S., Hashimoto, Y. & Kon, Y. Characterization of mouse mediastinal fat-associated lymphoid clusters. Cell Tissue Res. 357, 731–741 (2014).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Price, A.E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107, 11489–11494 (2010).
Neill, D.R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Spits, H. et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Randall, T.D., Carragher, D.M. & Rangel-Moreno, J. Development of secondary lymphoid organs. Annu. Rev. Immunol. 26, 627–650 (2008).
Roozendaal, R. & Mebius, R.E. Stromal cell-immune cell interactions. Annu. Rev. Immunol. 29, 23–43 (2011).
Ruddle, N.H. & Akirav, E.M. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J. Immunol. 183, 2205–2212 (2009).
Ngo, V.N. et al. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999).
Krautler, N.J. et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 150, 194–206 (2012).
Matsumoto, M. et al. Distinct roles of lymphotoxin α and the type I tumor necrosis factor (TNF) receptor in the establishment of follicular dendritic cells from non-bone marrow-derived cells. J. Exp. Med. 186, 1997–2004 (1997).
Ansel, K.M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Fu, Y.X., Huang, G., Wang, Y. & Chaplin, D.D. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α-dependent fashion. J. Exp. Med. 187, 1009–1018 (1998).
Yang, Y. et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc. Natl. Acad. Sci. USA 109, 5388–5393 (2012).
Yang, Y. et al. Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proc. Natl. Acad. Sci. USA 109, 5382–5387 (2012).
Brennan, P.J., Brigl, M. & Brenner, M.B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13, 101–117 (2013).
Marshall, J.L. et al. The capsular polysaccharide Vi from Salmonella typhi is a B1b antigen. J. Immunol. 189, 5527–5532 (2012).
Gil-Cruz, C. et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. USA 106, 9803–9808 (2009).
Foote, J.B. & Kearney, J.F. Generation of B cell memory to the bacterial polysaccharide α-1,3 dextran. J. Immunol. 183, 6359–6368 (2009).
Cascalho, M., Ma, A., Lee, S., Masat, L. & Wabl, M. A quasi-monoclonal mouse. Science 272, 1649–1652 (1996).
Marshall, J.L. et al. Early B blasts acquire a capacity for Ig class switch recombination that is lost as they become plasmablasts. Eur. J. Immunol. 41, 3506–3512 (2011).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Fütterer, A., Mink, K., Luz, A., Kosco-Vilbois, M.H. & Pfeffer, K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Sun, Z. et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Koni, P.A. & Flavell, R.A. A role for tumor necrosis factor receptor type 1 in gut-associated lymphoid tissue development: genetic evidence of synergism with lymphotoxin β. J. Exp. Med. 187, 1977–1983 (1998).
Pabst, O. et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J. Immunol. 177, 6824–6832 (2006).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Kamada, N., Seo, S.U., Chen, G.Y. & Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Ji, Y. et al. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J. Biol. Chem. 287, 24378–24386 (2012).
Ji, Y. et al. Activation of natural killer T cells promotes M2 macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J. Biol. Chem. 287, 13561–13571 (2012).
Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 (2012).
Schipper, H.S. et al. Natural killer T cells in adipose tissue prevent insulin resistance. J. Clin. Invest. 122, 3343–3354 (2012).
Wu, L. et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl. Acad. Sci. USA 109, E1143–E1152 (2012).
Fukuyama, S. et al. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3−CD4+CD45+ cells. Immunity 17, 31–40 (2002).
Bénézech, C. et al. Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity 37, 721–734 (2012).
Gil-Ortega, M. et al. Native adipose stromal cells egress from adipose tissue in vivo: evidence during lymph node activation. Stem Cells 31, 1309–1320 (2013).
Kontoyiannis, D., Pasparakis, M., Pizarro, T.T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Casola, S. et al. Tracking germinal center B cells expressing germ-line immunoglobulin γ1 transcripts by conditional gene targeting. Proc. Natl. Acad. Sci. USA 103, 7396–7401 (2006).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Grivennikov, S.I. et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005).
Jenkins, S.J. et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011).
Acknowledgements
We thank the personnel of the Biomedical Services Unit of the University of Birmingham for animal colony care; E. Jenkinson for support; G. Anderson, P. Lane, and A. Rot for comments on the manuscript; C. Buckley for facilitating animal procedures; R. Bird for cell sorting; K. Pfeffer (Heinrich Heine University) for Ltbr−/− mice; G. Eberl (Institut Pasteur) for tissues from germ-free mice; D. Finke (University of Basel) for tissues from Cxcr5−/− mice; A. Diefenback (University of Mainz) for tissues from Id2CreErt2/+Gt(ROSA)26SorYFP/+Gata3f/f mice; and the US National Institutes of Health Tetramer Facility for CD1d-PBS-57 loaded tetramers. Supported by the European Union Framework Programme 7 integrated project INFLACARE (J.H.C.); the Biotechnology and Biological Sciences Research Council (BB/K004900/1 to J.H.C.) and the College of Medical and Dental Sciences of the University of Birmingham (J.H.C.); the American Asthma Foundation, the UK Medical Research Council and the Wellcome Trust (100963/Z/13/Z) for work in the A. McKenzie laboratory; the Biotechnology and Biological Sciences Research Council institute synergy programme, the European Research Council (280307) and the Agency for Science, Technology and Research (to Y.L.) for work in the M. Veldhoen laboratory; Deutsche Forschungsgemeinschaf (NE1466/2-1), the Russian Science Foundation (14-50-00060) and the Russian Foundation for Basic Research (13-04-40268 to A.A.K.) for work in the S. Nedospasov laboratory; the European Research Council (340217-MCs_inTEST) for work in the G. Kollias laboratory; the Medical Research Council (S.N.); the UK Medical Research Council (MRC/K01207X/1 to L.H.J.); and the Lifelong Health and Wellbeing cross council initiative (Topjabs G1001390 for K.M.T., Y.Z. and J.M.).
Author information
Authors and Affiliations
Contributions
C.B., N.-T.L., J.A.W., A.A.K., Y.L., K.N., Y.Z., S.N., L.H.J. and J.H.C. designed and performed the research and collected and analyzed the data; A.F.-L., A.M., J.M., F.B., G.B., K.M., J.E.A., M.G., G.K., A.F.C., D.R.W., K.M.T., N.D.J., M.V., S.A.N. and A.N.J.M. facilitated the research; and C.B. and J.H.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 FALC morphology.
Whole mount immunofluorescence staining showing a representative array of FALCs harvested from the mesenteries of a single C57BL/6J (WT) mouse with CD11b+ myeloid cells (blue), CD45+ hematopoietic cells (green), IgM+ B cells (red), and CD4+ T cells (white) on the top row and without CD45 staining on the bottom row. Pictures are representative of 6 mice. Scale bar 50 μm.
Supplementary Figure 2 B cell activation in FALCs upon peritoneal immunological challenge.
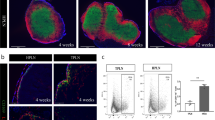
(a) Flow cytometry of the mesenteries of QM/QM (IgHNP/NP Igκ−/−) mice 24 hours post PBS (left panel) and NP-Ficoll (right panel) IP injection. In the CD45+ hematopoietic fraction, the different B cell subsets were identified according to the expression of CD19 and CD11b (first row), CD19 and CD5 (second row). The percentages of NP+Ki67+ cells in the B2 (CD19+CD11b−CD5-), B1c (CD19+CD11b−CD5+), B1a (CD19+CD11b+CD5+) and B1b (CD19+CD11b+CD5-) cell subsets are shown (third and forth row). Image representative of 2 independent experiments (n=6, 5). (b) Quantification of the percentage of NP+Ki67+ activated B cells in the mesenteries, peritoneal cavity lavage (PCL) and spleen of QM/QM mice 24 hours after PBS or NP-Ficoll injections. Data are representative of 2 independent experiments (n=6, 5). Each symbol represents an individual mouse. Mann-Whitney nonparametric two-tailed test, *p < 0.05, **p < 0.01, ***p< 0.001, ****p < 0.0001, and NS not significant. (c) Whole mount immunofluorescence staining showing FALCs from QM/QM mice after PBS or NP-Ficoll injection with CD11b+ myeloid cells (blue), CD45+ hematopoietic cells (green) and IgM+ B cells (red). Pictures representative of 8 clusters from 4 mice for each condition. Scale bar 50 μm.
Supplementary Figure 3 ILC2 cells in visceral AT and FALCs.
(a) Flow cytometry of the mesenteries showing CD45+Lin-c-Kit+Sca-1+IL-7Rα+ ILC2 in WT (first row) and Rag2−/− (second row) mice (NK1.1 and CD4 present in Lin cocktail). Data representative of 4 mice (b) Flow cytometric analysis of the mesenteries showing CD45+Lin-Sca-1+NK1.1- ILC2 and CD45+Lin-Sca-1-+NK1.1+ NK cells in Rag2−/− mice. Data representative of 4 mice (c) Whole mount immunofluorescence staining showing FALCs in Rag2−/− with CD11b+ myeloid cells (blue) and CD90+ ILC (green). The picture is representative of 8 clusters from 4 mice. Scale bar 50 μm.
Supplementary Figure 4 Myeloid cells are recruited to FALCs when inflammation is elicited.
Whole mount immunofluorescence staining showing FALCs containing CD11b+ myeloid cells (blue) and Ly6C+ monocytes (first row, green), Gr1+ granulocytes (second row, green) or F4/80+ macrophages (third row, green) after zymosan injection. Pictures are representative of 8 clusters from 4 mice. Scale bar 50 μm.
Supplementary Figure 5 IFN-γ is not necessary for the formation of FALCs when inflammation is elicited.
Number of clusters found in the mesenteries of Ifng−/− mice and whole mount immunofluorescence staining showing FALCs with CD11b+ myeloid cells (blue), CD45+ hematopoietic cells (green), IgM+ B cells (red), and CD4+ T cells (white) 72 hours after IP injection of PBS or zymosan. Data corrrespond to control (n=6) and treated (n=4) mice pooled from 2 independent experiments. Each symbol represents an individual mouse Mann-Whitney nonparametric two-tailed test, **p < 0.01. Pictures are representative of 8 clusters from 4 mice. Scale bar 50 μm. Scale bar 50 μm.
Supplementary Figure 6 Partial ‘rescue’ of inflamation-induced FALC formation with IL-4c in the absence of TNF signaling.
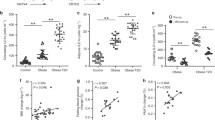
(a) Number of clusters found in the mesenteries of C57BL/6J (WT) mice and whole mount immunofluorescence staining showing FALCs with CD11b+ myeloid cells (blue), CD45+ hematopoietic cells (green), IgM+ B cells (red), and CD4+ T cells (white) four days after PBS, TNF, IL-4c and both TNF and IL4c injections. Data correspond to control (n=6) and TNF (n=6), IL-4c (n=7) and TNF+IL-4c (n=7) treated mice, pooled from 2 independent experiments. Mann-Whitney nonparametric two-tailed test, PBS and TNF p=0.4248, PBS and IL-4c p=0.4156, PBS and TNF+IL-4c p= 0.7964. Pictures are representative of 8 clusters from 4 mice for each condition. (b) Number of clusters found in the mesenteries of C57BL/6J (WT), Il4r a−/− and Tnfrsf1a−/− Tnfrsf1b−/− mice and whole mount immunofluorescence staining as in a, 4 days after PBS, zymosan, zymosan+TNF, and zymosan+IL-4c injections. Data correspond to PBS (n=13), zymosan (n=15) and zymosan+TNF (n=9) treated WT mice pooled from 3 independent experiments, and to PBS (n=7), zymosan (n=7) and zymosan+TNF (n=8) treated Il4ra−/− mice pooled from 2 independent experiments, and to zymosan (n=7) and zymosan+IL-4c (n=7) treated Tnfrsf1a−/− Tnfrsf1b−/− mice pooled from 2 independent experiments. Each symbol represents an individual mouse. Mann-Whitney nonparametric two-tailed test. *p < 0.05, **p < 0.01, ***p< 0.001 and ****p <0.0001, NS not significant. Pictures are representative of 8 clusters from 4 mice for each condition. Scale bar 50 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 6057 kb)
Rights and permissions
About this article
Cite this article
Bénézech, C., Luu, NT., Walker, J. et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol 16, 819–828 (2015). https://doi.org/10.1038/ni.3215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3215
This article is cited by
-
Obese visceral fat tissue inflammation: from protective to detrimental?
BMC Medicine (2022)
-
Harnessing murine models of Crohn's disease ileitis to advance concepts of pathophysiology and treatment
Mucosal Immunology (2022)
-
CXCL13 is expressed in a subpopulation of neuroendocrine cells in the murine trachea and lung
Cell and Tissue Research (2022)
-
Immunofibroblasts regulate LTα3 expression in tertiary lymphoid structures in a pathway dependent on ICOS/ICOSL interaction
Communications Biology (2022)
-
Spatiotemporally specific roles of TLR4, TNF, and IL-17A in murine endotoxin-induced inflammation inferred from analysis of dynamic networks
Molecular Medicine (2021)