Abstract
Interferon-γ (IFN-γ) primes macrophages for enhanced microbial killing and inflammatory activation by Toll-like receptors (TLRs), but little is known about the regulation of cell metabolism or mRNA translation during this priming. We found that IFN-γ regulated the metabolism and mRNA translation of human macrophages by targeting the kinases mTORC1 and MNK, both of which converge on the selective regulator of translation initiation eIF4E. Physiological downregulation of mTORC1 by IFN-γ was associated with autophagy and translational suppression of repressors of inflammation such as HES1. Genome-wide ribosome profiling in TLR2-stimulated macrophages showed that IFN-γ selectively modulated the macrophage translatome to promote inflammation, further reprogram metabolic pathways and modulate protein synthesis. These results show that IFN-γ–mediated metabolic reprogramming and translational regulation are key components of classical inflammatory macrophage activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Hu, X. & Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009).
Stark, G.R. & Darnell, J.E. Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514 (2012).
Hu, X. et al. IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24, 563–574 (2006).
Hu, X. et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-γ pathways. Immunity 29, 691–703 (2008).
Ostuni, R. et al. Latent enhancers activated by stimulation in differentiated cells. Cell 152, 157–171 (2013).
Qiao, Y. et al. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and Toll-like receptor signaling. Immunity 39, 454–469 (2013).
Piccirillo, C.A., Bjur, E., Topisirovic, I., Sonenberg, N. & Larsson, O. Translational control of immune responses: from transcripts to translatomes. Nat. Immunol. 15, 503–511 (2014).
Wan, Y. et al. Interleukin-1 receptor-associated kinase 2 is critical for lipopolysaccharide-mediated post-transcriptional control. J. Biol. Chem. 284, 10367–10375 (2009).
Xu, H. et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 13, 642–650 (2012).
Herdy, B. et al. Translational control of the activation of transcription factor NF-κB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat. Immunol. 13, 543–550 (2012).
Colina, R. et al. Translational control of the innate immune response through IRF-7. Nature 452, 323–328 (2008).
Larsson, O. et al. Apoptosis resistance downstream of eIF4E: posttranscriptional activation of an anti-apoptotic transcript carrying a consensus hairpin structure. Nucleic Acids Res. 34, 4375–4386 (2006).
Ivashkiv, L.B. & Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Mohr, I. & Sonenberg, N. Host translation at the nexus of infection and immunity. Cell Host Microbe 12, 470–483 (2012).
Schoggins, J.W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Kroczynska, B., Mehrotra, S., Arslan, A.D., Kaur, S. & Platanias, L.C. Regulation of interferon-dependent mRNA translation of target genes. J. Interferon Cytokine Res. 34, 289–296 (2014).
Mukhopadhyay, R., Jia, J., Arif, A., Ray, P.S. & Fox, P.L. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem. Sci. 34, 324–331 (2009).
Laplante, M. & Sabatini, D.M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Weichhart, T. et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 (2008).
Turnquist, H.R. et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 115, 4758–4769 (2010).
Hu, X., Chakravarty, S.D. & Ivashkiv, L.B. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 226, 41–56 (2008).
Joshi, B. et al. Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J. Biol. Chem. 270, 14597–14603 (1995).
Hsieh, A.C. et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012).
Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722–737 (2013).
Zoncu, R. et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678–683 (2011).
Bar-Peled, L., Schweitzer, L.D., Zoncu, R. & Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 (2012).
Hara, K. et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 273, 14484–14494 (1998).
Taylor, M.W. & Feng, G.S. Relationship between interferon-γ, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 5, 2516–2522 (1991).
Mellor, A.L. & Munn, D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774 (2004).
Powell, J.D., Pollizzi, K.N., Heikamp, E.B. & Horton, M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39–68 (2012).
Ji, J.D. et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-γ in human osteoclast precursors. J. Immunol. 183, 7223–7233 (2009).
Ingolia, N.T., Ghaemmaghami, S., Newman, J.R. & Weissman, J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 (2009).
Thoreen, C.C. et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 (2012).
Kristensen, A.R., Gsponer, J. & Foster, L.J. Protein synthesis rate is the predominant regulator of protein expression during differentiation. Mol. Syst. Biol. 9, 689 (2013).
Han, J.M. et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149, 410–424 (2012).
Iliopoulos, D., Hirsch, H.A. & Struhl, K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706 (2009).
Tattoli, I. et al. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11, 563–575 (2012).
Ivanov, S.S. & Roy, C.R. Pathogen signatures activate a ubiquitination pathway that modulates the function of the metabolic checkpoint kinase mTOR. Nat. Immunol. 14, 1219–1228 (2013).
O'Neill, L.A. & Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493, 346–355 (2013).
Ganeshan, K. & Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 32, 609–634 (2014).
Cortese, M., Sinclair, C. & Pulendran, B. Translating glycolytic metabolism to innate immunity in dendritic cells. Cell Metab. 19, 737–739 (2014).
O'Neill, L.A. Glycolytic reprogramming by TLRs in dendritic cells. Nat. Immunol. 15, 314–315 (2014).
Martinez, F.O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Mercalli, A. et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology 140, 179–190 (2013).
Byles, V. et al. The TSC-mTOR pathway regulates macrophage polarization. Nat. Commun. 4, 2834 (2013).
Cheng, S.C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Lemaitre, B. & Girardin, S.E. Translation inhibition and metabolic stress pathways in the host response to bacterial pathogens. Nat. Rev. Microbiol. 11, 365–369 (2013).
Lo, W.S. et al. Human tRNA synthetase catalytic nulls with diverse functions. Science 345, 328–332 (2014).
Ingolia, N.T., Brar, G.A., Rouskin, S., McGeachy, A.M. & Weissman, J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
Robinson, M.D., McCarthy, D.J. & Smyth, G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Acknowledgements
We thank members of the Weill Cornell Medical College Genomics Core for advice about RNA-Seq, J. Schulze (UC Davis Proteomics Core) for advice about amino acid measurements, B. Zhao and L. Donlin for review of the manuscript, K. Park-Min (Hospital for Special Surgery, New York, New York, USA) for providing Myc inhibitor and siRNA oligos, and S. Park and Y. Qiao for discussion about bioinformatic analysis. This work was supported by the NIH (grants to L.B.I. and C.M.R.), the Leonard Tow Foundation (grant to the David Z. Rosensweig Genomics Research Center), the Greenberg Medical Research Institute, the Starr Foundation (grant to C.M.R.) and the European Commission (EU PITN-GA-2012-316861 to Y.Z.).
Author information
Authors and Affiliations
Contributions
X.S. designed and conducted experiments, analyzed data and prepared the manuscript; Y.Y. performed polysome-profiling and ribosome-profiling experiments and analyzed data; Y.Z. analyzed ribosome-profiling, RNA-Seq and miRNA-Seq data; E.G.G. analyzed ribosome-profiling and RNA-Seq data; J.R.C. and H.L. performed liquid chromatography–mass spectrometry experiments and analyzed data; X.H., G.R. and C.M.R. provided advice about experiments and data analysis and contributed to manuscript preparation; L.B.I. conceived and supervised experiments and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
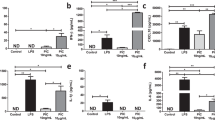
Supplementary Figure 1 IFN-γ–mediated HES1 downregulation is independent of accelerated protein decay.
(a) qPCR analysis of HES1 mRNA (upper panel) and HEY1 mRNA (lower panel) in control and IFN-γ–primed macrophages stimulated with LPS (10 ng/ml) or Pam3CSK4 (10 ng/ml) for 3 h; results were normalized relative to the levels of GAPDH mRNA. (b) Cumulative data from 23 independent donors of relative expression of HES1 mRNA in control and IFN-γ–primed macrophages stimulated with Pam3CSK4 (10 ng/ml) for 4 h. HES1 mRNA expression in IFN-γ−primed macrophages relative to control macrophages (set at 1) for each individual donor is depicted, and error bars represent s.e.m. P > 0.05 by two-tailed paired Student’s t-test. (c) IFN-γ does not accelerate HES1 protein degradation. Immunoblot analysis of HES1 in control and IFN-γ–primed macrophages stimulated with Pam3CSK4 (10 ng/ml) for 4 h. Cells were treated with cycloheximide (CHX, 20 μg/ml) to stop new protein synthesis, and HES1 protein degradation was followed over a time course as indicated; p38α served as a loading control. (d) Inhibition of proteasomes does not reverse the suppressive effect of IFN-γ on HES1 protein expression. Immunoblot analysis of HES1 (upper panel) in control and IFN-γ–primed macrophages stimulated with Pam3CSK4 (10 ng/ml) for 3 h and then treated with proteasome inhibitor MG132 (10 μM) for a 2-h time course; p38α served as a loading control. Immunoblot analysis of I-κBα (lower panel) in the same experiment confirmed the efficacy of proteasome inhibition. (e) Inhibition of lysosomal function does not reverse the suppressive effect of IFN-γ on HES1 protein expression. Immunoblot analysis of HES1 and LC3A in control and IFN-γ–primed macrophages pretreated with Bafilomycin A1 (BafA1, 100 nM) for 30 min and then stimulated with Pam3CSK4 (10 ng/ml) for 4 h; p38α served as a loading control. Increased LC3A expression confirmed the efficacy of lysosome inhibition.
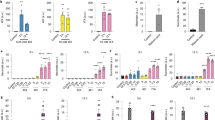
Supplementary Figure 2 IFN-γ suppresses translation of the established MNK-eIF4E targets I-κBα and IRF8.
(a) Schematic representation of the MAPK-MNK and mTORC1 signaling pathways that target eIF4E to promote translation. (b) Real-time PCR analysis of NFKBIA (I-κBα) mRNA (left panel) in control and IFN-γ–primed macrophages. Immunoblot analysis of I-κBα (right panel) in nuclear extracts of control or IFN-γ–primed macrophages; TBP served as a loading control. IFN-γ–mediated suppression of I-κBα protein expression reflects suppression of upstream MNK-eIF4E signaling. (c) Immunoblot analysis of I-κBα in macrophages pretreated for 30 min with DMSO or increasing concentrations of the MNK inhibitor CGP57380 (5 μM, 10 μM, 20 μM) and then stimulated with Pam3CSK4 (10 ng/ml) for 4 h; p38α served as a loading control. (d) Polysome-shift analysis of IRF8 mRNA. (e) Immunoblot and qPCR confirmation of efficacy of siRNA-mediated knockdown of MNK expression in primary human macrophages. (f) qPCR analysis of HES1 mRNA in human primary macrophages transfected with scrambled control siRNA or MKNK1- and MKNK2-specific siRNA for 72 h and then stimulated or not stimulated with Pam3CSK4 (10 ng/ml). (g) Immunoblot analysis of phosphorylated (p-) p38, p-Erk, p-Akt in control and IFN-γ–primed macrophages pretreated with okadaic acid (OA) (40 nm) for 30 min before stimulation with Pam3CSK4 for 0–30 min; p38α served as a loading control.
Supplementary Figure 3 Rapamycin promotes inflammatory cytokine production in human macrophages and minimally affects HES1 mRNA expression.
(a) Immunoblot analysis of phosphorylated (p-) 4E-BP1 in human primary monocyte–derived dendritic cells (hMo-DCs) treated with IFN-γ for 24 h before stimulation with Pam3CSK4 (10 ng/ml) or LPS (10 ng/ml) for 1 h. p38α served as a loading control. (b) Cytometric Bead Array (CBA) analysis of TNF, IL-6 and IL-10 in culture supernatants of human primary macrophages pretreated with vehicle control DMSO or rapamycin (500 nM) for 30 min and then stimulated with Pam3CSK4 for 6 h. (c) Real-time PCR analysis of HES1 mRNA in human primary macrophages pretreated for 30 min with vehicle control DMSO (labeled as 0) or increasing concentrations of mTORC1 inhibitor rapamycin (0.5 µM, 1 µM), and then stimulated with Pam3CSK4 (10 ng/ml) for 4 h. (d) Schematic representation of binary signals required for mTORC1 activation. Two upstream signals lead to activation of mTORC1: amino acid pathway and growth factors/inflammatory stimuli pathway. (e,f) Heat maps (log10 scale) of intracellular (e) and extracellular (f) tryptophan and its downstream catabolites in the IDO-mediated degradation pathway. Panels e and f show triplicate determinants from a representative experiment.
Supplementary Figure 4 Baseline mTORC1 activity in macrophages is dependent on serum and M-CSF.
(a) Immunoblot analysis of phosphorylated (p-) 4E-BP1 in macrophages cultured for 24 h with 5 ng or 20 ng M-CSF and with 2.5% or 10% serum (FBS); p38α served as a loading control. (b) Inhibition of M-CSFR signaling using imatinib decreases basal p-4E-BP phosphorylation. Immunoblot analysis of phosphorylated (p-) 4E-BP1 in macrophages treated with vehicle control DMSO (labeled as 0) or imatinib (300 nM) for 0–6 h; p38α served as a loading control. (c) MTT assay of cultures of control or IFN-γ–primed macrophages. Data from eight independent blood donors is shown. (d) Immunoblot analysis of HES1 in human primary macrophages pretreated with vehicle control DMSO or Myc inhibitor 10058-F4 (60 µM) for 30 min and then stimulated with Pam3CSK4 (10 ng/ml) for 0–4 h; p38α served as a loading control. (e) Immunoblot analysis of HES1 and phosphorylated (p-) 4E-BP1 (upper panel) in macrophages transfected with scrambled control small interfering RNA (siRNA) or Myc-specific siRNA for 24 h and then stimulated or not stimulated with Pam3CSK4 (10 ng/ml) for 4 h; 4E-BP1 and p38α served as loading controls. Immunoblot analysis of c-Myc (lower panel) in nuclear and cytosol extracts of macrophages confirmed the efficacy of siRNA-mediated knockdown; TBP and Akt served as loading controls.
Supplementary Figure 5 Ribosome profiling replicates are highly reproducible.
(a) Schematic of the ribosome profiling experimental design. RNA-Seq, RNA sequencing. (b) Correlation plots from two independent ribosome-profiling experiments as described in a. The Pearson correlation value was calculated by GraphPrism. (c) Frequency distribution of the ratio of TE in control and IFN-γ–primed macrophages (left panel); ΔTE = log2(TEIFN-γ/TEcontrol). Number of genes identified as downregulated (blue) and upregulated (red) with different cutoffs (z-score = 1.5-fold and 2-fold) are shown in the table on the right. Data were generated from a merged data set of two biological replicates. (d) Ribosome-protected fragment (RPF) read density profiles for HES1 in control (yellow) and IFN-γ–primed (purple) macrophages. Ribosomal occupancy was diminished in coding exons, consistent with decreased protein observed by immunoblotting. However, ribosomal occupancy in exons corresponding to the 5ʹ UTR did not change, suggesting ribosome stalling in potential open reading frames upstream of the initiator AUG. This intact ribosomal occupancy in the 5ʹ UTR is consistent with the lesser polysome shift to monosomal fractions shown for HES1 mRNA in Fig. 1d.
Supplementary Figure 6 Genome-wide functional annotation reveals concordant pattern of canonical pathway enrichment in biological replicates.
(a) Ingenuity Pathway Analysis (IPA) of canonical pathways most significantly enriched in genes regulated by IFN-γ at the level of ribosome protected fragments (RPFs). We generated the heat map by comparing independent analyses of a combined data set (replicate 1 and replicate 2) and individual analysis of replicate 1 and replicate 2. Left panel shows activation z-score calculated by IPA; right panel shows significance by P value. (b) Heat map showing changes in RPF, RNA and TE of 35 tRNA genes. Data were generated from a merged data set from two biological replicates.
Supplementary Figure 7 Gene Ontology analysis reveals IFN-γ–mediated translational control of metabolic and immune-system genes.
(a,b) Pie charts showing functional classification of genes identified by Gene Ontology analysis of genes whose translation was suppressed (a) or increased (b) by IFN-γ. The analysis was done with the PANTHER classification system (www.pantherdb.org). Data shown in this figure were generated from a merged data set from two biological replicates.
Supplementary Figure 8 Enrichment of metabolic pathways in genes whose translational efficiency was upregulated or downregulated by IFN-γ.
(a) Ingenuity Pathway Analysis (IPA) of canonical pathways most significantly enriched in metabolic genes regulated by IFN-γ at the level of translation efficiency (TE) (corresponding to blue wedges in pie charts in Supplementary Fig. 7). We generated the heat map by comparing independent analyses of TE-upregulated and TE-downregulated metabolic gene sets. (b) Immunoblot analysis of phosphorylated (p-) eIF2α in control and IFN-γ–primed macrophages stimulated with Pam3CSK4 (10 ng/ml) for 0–4 h; total eIF2α and p38α served as loading controls. (c) Working model of selective regulation of translation by IFN-γ. IFN-γ inhibits TLR-induced activation of MAPK signaling pathways, resulting in diminished eIF4E phosphorylation and activity. IFN-γ also inhibits activation of the metabolic regulator mTORC1 through suppression of amino acid and growth factor pathways, resulting in decreased p-4E-BPs and eIF4E activity and altered translation. Metabolic and translational control are integrated, as metabolic changes affected translation and translational fine-tuning affected metabolism-related mRNAs.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 3 and 4 (PDF 1020 kb)
Supplementary Table 1
Metabolic genes whose translation was increased or suppressed by IFN-γ Genes contained in blue wedge in pie chart in Supplementary Fig. 7a-b (XLSX 2411 kb)
Supplementary Table 2
Immune genes whose translation was increased or suppressed by IFN-γ Genes contained in red wedge in pie chart in Supplementary Fig. 7a-b (XLSX 2539 kb)
Rights and permissions
About this article
Cite this article
Su, X., Yu, Y., Zhong, Y. et al. Interferon-γ regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat Immunol 16, 838–849 (2015). https://doi.org/10.1038/ni.3205
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3205
This article is cited by
-
The impact of prior exposure to hypoglycaemia on the inflammatory response to a subsequent hypoglycaemic episode
Cardiovascular Diabetology (2024)
-
Translational regulation and protein-coding capacity of the 5′ untranslated region of human TREM2
Communications Biology (2023)
-
Evaluating ribosomal frameshifting in CCR5 mRNA decoding
Nature (2022)
-
Nanoparticles with ultrasound-induced afterglow luminescence for tumour-specific theranostics
Nature Biomedical Engineering (2022)
-
Macrophages disseminate pathogen associated molecular patterns through the direct extracellular release of the soluble content of their phagolysosomes
Nature Communications (2022)