Abstract
Foxm1 is known as a typical proliferation-associated transcription factor. Here we found that Foxm1 was essential for maintenance of the quiescence and self-renewal capacity of hematopoietic stem cells (HSCs) in vivo in mice. Reducing expression of FOXM1 also decreased the quiescence of human CD34+ HSCs and progenitor cells, and its downregulation was associated with a subset of myelodysplastic syndrome (MDS). Mechanistically, Foxm1 directly bound to the promoter region of the gene encoding the receptor Nurr1 (Nr4a2; called 'Nurr1' here), inducing transcription, while forced expression of Nurr1 reversed the loss of quiescence observed in Foxm1-deficient cells in vivo. Thus, our studies reveal a previously unrecognized role for Foxm1 as a critical regulator of the quiescence and self-renewal of HSCs mediated at least in part by control of Nurr1 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Reya, T., Morrison, S.J., Clarke, M.F. & Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Cheung, T.H. & Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340 (2013).
Pietras, E.M., Warr, M.R. & Passegue, E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 195, 709–720 (2011).
Kalin, T.V., Ustiyan, V. & Kalinichenko, V.V. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle 10, 396–405 (2011).
Costa, R.H. FoxM1 dances with mitosis. Nat. Cell Biol. 7, 108–110 (2005).
Laoukili, J. et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 7, 126–136 (2005).
Ye, H. et al. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol. Cell. Biol. 17, 1626–1641 (1997).
Kalin, T.V. et al. Forkhead Box m1 transcription factor is required for perinatal lung function. Proc. Natl. Acad. Sci. USA 105, 19330–19335 (2008).
Ustiyan, V. et al. Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus. Dev. Biol. 336, 266–279 (2009).
Wang, X., Kiyokawa, H., Dennewitz, M.B. & Costa, R.H. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc. Natl. Acad. Sci. USA 99, 16881–16886 (2002).
Ackermann Misfeldt, A., Costa, R.H. & Gannon, M. Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 57, 3069–3077 (2008).
Ye, H., Holterman, A.X., Yoo, K.W., Franks, R.R. & Costa, R.H. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol. Cell. Biol. 19, 8570–8580 (1999).
Koo, C.Y., Muir, K.W. & Lam, E.W. FOXM1: from cancer initiation to progression and treatment. Biochim. Biophys. Acta 1819, 28–37 (2012).
Xue, L., Chiang, L., He, B., Zhao, Y.Y. & Winoto, A. FoxM1, a forkhead transcription factor is a master cell cycle regulator for mouse mature T cells but not double positive thymocytes. PLoS ONE 5, e9229 (2010).
Ren, X. et al. Forkhead box M1 transcription factor is required for macrophage recruitment during liver repair. Mol. Cell. Biol. 30, 5381–5393 (2010).
Sirin, O., Lukov, G.L., Mao, R., Conneely, O.M. & Goodell, M.A. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat. Cell Biol. 12, 1213–1219 (2010).
Kisanuki, Y.Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230–242 (2001).
Ficara, F., Murphy, M.J., Lin, M. & Cleary, M.L. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell 2, 484–496 (2008).
Socolovsky, M. et al. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood 98, 3261–3273 (2001).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Adolfsson, J. et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005).
Ding, L., Saunders, T.L., Enikolopov, G. & Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Butler, J.M. et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264 (2010).
Kobayashi, H. et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046–1056 (2010).
Kühn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Ma, R.Y. et al. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J. Cell Sci. 118, 795–806 (2005).
Wang, I.C. et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell. Biol. 25, 10875–10894 (2005).
Wang, I.C. et al. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J. Biol. Chem. 283, 20770–20778 (2008).
Yilmaz, O.H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Qian, Z., Chen, L., Fernald, A.A., Williams, B.O. & Le Beau, M.M. A critical role for Apc in hematopoietic stem and progenitor cell survival. J. Exp. Med. 205, 2163–2175 (2008).
Harrison, D.E., Stone, M. & Astle, C.M. Effects of transplantation on the primitive immunohematopoietic stem cell. J. Exp. Med. 172, 431–437 (1990).
Xue, S. et al. RNA regulons in Hox 5′ UTRs confer ribosome specificity to gene regulation. Nature 517, 33–38 (2015).
Signer, R.A., Magee, J.A., Salic, A. & Morrison, S.J. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509, 49–54 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Helin, K. Regulation of cell proliferation by the E2F transcription factors. Curr. Opin. Genet. Dev. 8, 28–35 (1998).
Wierstra, I. & Alves, J. Despite its strong transactivation domain, transcription factor FOXM1c is kept almost inactive by two different inhibitory domains. Biol. Chem. 387, 963–976 (2006).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Lacorazza, H.D. et al. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell 9, 175–187 (2006).
Nimer, S.D. MDS: a stem cell disorder–but what exactly is wrong with the primitive hematopoietic cells in this disease? Hematology 43–51 (2008).
Pellagatti, A. et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia 24, 756–764 (2010).
Xie, Z. et al. Foxm1 transcription factor is required for maintenance of pluripotency of P19 embryonal carcinoma cells. Nucleic Acids Res. 38, 8027–8038 (2010).
Wang, Z. et al. FoxM1 in tumorigenicity of the neuroblastoma cells and renewal of the neural progenitors. Cancer Res. 71, 4292–4302 (2011).
Zhang, N. et al. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell 20, 427–442 (2011).
Wierstra, I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv. Cancer Res. 118, 97–398 (2013).
Qian, Z., Chen, L., Fernald, A.A., Williams, B.O. & Le Beau, M.M. A critical role for Apc in hematopoietic stem and progenitor cell survival. J. Exp. Med. 205, 2163–2175 (2008).
Hou, Y. et al. FHL2 regulates hematopoietic stem cell functions under stress conditions. Leukemia 3, 615–624 (2014).
Irizarry, R.A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Zhang, C.C., Kaba, M., Iizuka, S., Huynh, H. & Lodish, H.F. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood 111, 3415–3423 (2008).
Acknowledgements
We thank M.A. Goodell (Baylor College of Medicine) for the plasmid MSCV-FlagNurr1. Supported by the US National Institutes of Health (RO1 CA140979 to Z.Q.).
Author information
Authors and Affiliations
Contributions
Y.Ho., W.L., Y.S., L.L., Y.Hu. and Z.Z. performed research; T.Z., D.P., J.G.Q., W.W. and Y.Z. provided advice, reagents and/or analytic tools; Y.Ho. and Z.Q. designed the research and performed data analysis; and Y.Ho., D.P., J.G.Q. and Z.Q. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
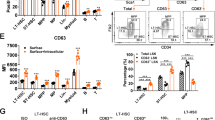
Supplementary Figure 1 Foxm1 loss leads to bone marrow hypocellularity.
(a-b) Analysis of ablation of Foxm1 as determined by semiquantitative PCR analysis of genomic DNA (a) as well as qRT-PCR analysis of mRNAs (b) from BM cells from both Foxm1fl/fl Tie2-Cre and Foxm1fl/fl mice. (c) Histological analysis of hematoxylin and eosin-stained sternum from the representative Foxm1fl/fl Tie2-Cre and Foxm1fl/fl mice.
Supplementary Figure 2 Foxm1 loss leads to a decreased number of mature blood cells but does not interfere with lineage differentiation.
(a) Histograms represent the total number of myeloid cells (Mac-1+Gr-1+), B cells (B220+ IgM+), Megakaryocyte (CD41+) and Macrophage (F4/80+) in BM from Foxm1fl/fl Tie2-Cre and Foxm1fl/fl mice. Mean ± standard deviation (SD); n = 5. *, P<0.05. (b-e) Representative histograms show the frequency of myeloid cells (Mac-1+Gr+) (b), B cells (B220+IgM+) (c), Megakaryocyte (CD41+) (d) and Macrophage (F4/80+) (e) in BM from Foxm1fl/fl Tie2-Cre and Foxm1fl/fl mice. (f-i) The frequency and representative flow cytometric histograms of four differentiation stages of erythroblasts including R1 (CD71+Ter119–), R2 (CD71+Ter119+), R3 (CD71lowTer119+) and R4 (CD71–Ter119+) in BM cells (f,h) and spleen cells (g,i) from Foxm1fl/fl Tie2-Cre and Foxm1fl/fl mice at age of 6-8 weeks.
Supplementary Figure 3 Foxm1 is efficiently deleted in LSK cells from Foxm1fl/flMx1-Cre mice after injection of poly(I:C).
(a) Analysis of Foxm1 deletion as determined by semiquantitative PCR analysis of genomic DNA from LSK cells from Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice 3 weeks after 3 doses of pI-pC injection. (b) qRT-PCR analysis of mRNAs from LSK cells from both Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice 3 weeks after 3 doses of pI-pC injection. (c) The total number of BM cells were reduced in Foxm1fl/fl Mx1-Cre mice as compared to Foxm1fl/fl mice (n=5, P<0.05).
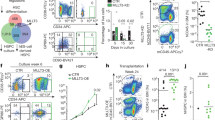
Supplementary Figure 4 Induction of Foxm1 deletion reduces HSC and HPC pools in Foxm1fl/flMx1-Cre chimeric mice.
(a) Comparison of engraftment frequency of Foxm1fl/fl Mx1-Cre (CD45.2+) or control Foxm1fl/fl BM cells (CD45.2+) in WT (CD45.1+) recipient mice by flow cytometric analysis of peripheral blood at 6 weeks after transplantation. (b) Total number of LSK, LSKCD34– and LT-HSC in BM from chimeric Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice at 6 weeks after pI-pC injection. (mean±SD, n=5). *, P<0.05. (c) Total number of HPC, CMP, GMP and MEP in BM from Foxm1fl/fl Mx1-Cre and Foxm1fl/fl chimeric mice at 6 weeks after pI-pC injection (mean±SD, n=5)*. P<0.05.
Supplementary Figure 5 Foxm1 deletion results in a decreased repopulation capacity but does not affect the lineage differentiation of BM cells.
(a-c) The ratio of donor-derived CD45.2+CD45.1– from Foxm1fl/flTie2-Cre or Foxm1fl/fl vs competitor-cell-derived CD45.1+CD45.2+ in myeloid cells, B cells, and T cells in PB from first and secondary recipient mice were analyzed (mean ± SD, n = 4-5). (d) Flow cytometric analysis of the frequency of LSK, LSK CD34– in the competitive repopulated recipient mice 4 months after secondary transplantation. (e) Flow cytometric analysis of CD45.2+CD45.1– and CD45.1+CD45.2+ cells in LSK and LSK CD34– stem cell–enriched population in the competitive repopulated recipient mice 4 months after secondary transplantation. Donor cells were from Foxm1fl/flTie2-Cre or Foxm1fl/fl. (f) Lineage differentiation in the recipient mice transplanted with BM cells from Foxm1fl/flTie2-Cre and Foxm1fl/fl chimeric mice. Histogram shows percentage of donor-derived (CD45.2+CD45.1–) myeloid cells (Gr-1+Mac+), B cells (B220+), and T cells (CD3+) in PB analyzed 4 months after the first and secondary transplantation (mean ± SD, n = 4-5).
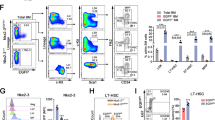
Supplementary Figure 6 Induction of Foxm1 deletion alters cell-cycle progression in HSCs and HPCs from Foxm1fl/flMx1-Cre mice and chimeric Foxm1fl/flMx1-Cre mice.
(a-c) The histograms depicting the cell cycle profile of HPCs (a), LSK cells (b) and LT-HSCs (c) in Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice (mean±SD, n=3). *, P<0.05, **, P<0.005. (d) The histogram depicting cell cycle status of LSK cells in Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice (mean±SD, n= 5). *, P<0.05, **, P<0.005. (e-g) The histograms depicting the cell cycle profile of HPCs (e), LSK cells (f) and LT-HSCs (g) in chimeric Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice (mean±SD, n=5). *, P<0.05; **, P<0.005; ***, P<0.0005. (h) The histogram depicting cell cycle status of LSK cells in chimeric Foxm1fl/fl Mx1-Cre and Foxm1fl/fl mice (mean±SD, n= 5). *, P<0.05; **, P<0.005.
Supplementary Figure 7 Loss of Foxm1 increases the apoptosis of HPCs and LSK cells under stress.
(a) Histogram shows the mean frequency of apoptosis of HPCs and LSK cells from Foxm1fl/fl Tie2-Cre and Foxm1fl/fl mice (mean ± SD; n = 11). (b) Histogram shows the mean frequency of apoptosis of HPCs and LSK cells from Foxm1fl/fl Tie2-Cre and Foxm1fl/fl chimeric mice (mean ± SD; n = 5). *, P<0.05. (c) Histogram shows the mean frequency of apoptosis of HPCs and LSK cells from Foxm1fl/fl Mx1-Cre and Foxm1fl/fl chimeric mice. (mean ± SD; n = 5). *, P<0.05.
Supplementary Figure 8 Expression of the genes encoding p21 and p27 is upregulated by Nurr1 overexpression.
(a) Doxycycline-induced expression of Flag-Nurr1 in BM cells from the chimeric mice. The Flag-Nurr1 expression in BM cells was determined by Western Blot. The Foxm1△/△ BM cells expressing pLVX-Tet-On and pLVX-tight-puro-Nurr1 or pLVX-tight-puro or Foxm1fl/fl BM cells expressing pLVX-Tet-On and pLVX-tight-puro from chimeric mice received two-week-induction of Doxycyline. (b) qRT-PCR analysis of p21, p27 and p16 expression in LSK cells. The LSK cells, isolated from Foxm1fl/fl Tie2-Cre or control Foxm1fl/fl mice, were infected with MSCV-puro-Nurr1 or control vector and cultured with Stemspan medium with cytokines. The cells were treated with Puromycin for 2 days before analysis. Gene expression was initially normalized to Actb expression. Values represent the fold changes in gene expression relative to that in Foxm1fl/fl LSK cells expressing control vector. *, P<0.05.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–4 (PDF 2324 kb)
Rights and permissions
About this article
Cite this article
Hou, Y., Li, W., Sheng, Y. et al. The transcription factor Foxm1 is essential for the quiescence and maintenance of hematopoietic stem cells. Nat Immunol 16, 810–818 (2015). https://doi.org/10.1038/ni.3204
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3204
This article is cited by
-
Cell-intrinsic factors governing quiescence vis-à-vis activation of adult hematopoietic stem cells
Molecular and Cellular Biochemistry (2023)
-
FOXM1 regulates glycolysis and energy production in multiple myeloma
Oncogene (2022)
-
Human alveolar progenitors generate dual lineage bronchioalveolar organoids
Communications Biology (2022)
-
Overexpression of aberrant Wnt5a and its effect on acquisition of malignant phenotypes in adult T-cell leukemia/lymphoma (ATL) cells
Scientific Reports (2021)
-
ACE2/ACE imbalance and impaired vasoreparative functions of stem/progenitor cells in aging
GeroScience (2021)