Abstract
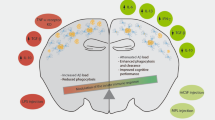
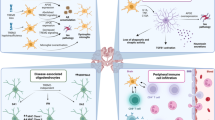
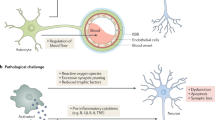
Alzheimer's disease (AD) is the world's most common dementing illness, affecting over 150 million patients. Classically AD has been viewed as a neurodegenerative disease of the elderly, characterized by the extracellular deposition of misfolded amyloid-β (Aβ) peptide and the intracellular formation of neurofibrillary tangles. Only recently has neuroinflammation emerged as an important component of AD pathology. Experimental, genetic and epidemiological data now indicate a crucial role for activation of the innate immune system as a disease-promoting factor. The sustained formation and deposition of Aβ aggregates causes chronic activation of the immune system and disturbance of microglial clearance functions. Here we review advances in the molecular understanding of the inflammatory response in AD that point to novel therapeutic approaches for the treatment of this devastating disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Marina Corral Spence/Nature Publishing Group


Marina Corral Spence/Nature Publishing Group

Marina Corral Spence/Nature Publishing Group
Similar content being viewed by others
References
Jack, C.R. Jr. et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Querfurth, H.W. & LaFerla, F.M. Alzheimer's disease. N. Engl. J. Med. 362, 329–344 (2010).
Bertram, L., Lill, C.M. & Tanzi, R.E. The genetics of Alzheimer disease: back to the future. Neuron 68, 270–281 (2010).
Mawuenyega, K.G. et al. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science 330, 1774–1774 (2010).
Walsh, D.M. et al. Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Hammer, N.D., Schmidt, J.C. & Chapman, M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. USA 104, 12494–12499 (2007).
Chapman, M.R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002).
Epstein, E.A. & Chapman, M.R. Polymerizing the fibre between bacteria and host cells: the biogenesis of functional amyloid fibres. Cell. Microbiol. 10, 1413–1420 (2008).
Monje, M.L., Toda, H. & Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765 (2003).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Sastre, M. et al. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of β-secretase. J. Neurosci. 23, 9796–9804 (2003).
In t' Veld, B.A. et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N. Engl. J. Med. 345, 1515–1521 (2001).
Breitner, J.C. The role of anti-inflammatory drugs in the prevention and treatment of Alzheimer's disease. Annu. Rev. Med. 47, 401–411 (1996).
Cagnin, A. et al. In-vivo measurement of activated microglia in dementia. Lancet 358, 461–467 (2001).
Yasuno, F. et al. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [11C]DAA1106. Psychiatry Res. 203, 67–74 (2012).
Lambert, J.-C. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat. Genet. 41, 1094–1099 (2009).
Karch, C.M., Cruchaga, C. & Goate, A.M. Alzheimer's disease genetics: from the bench to the clinic. Neuron 83, 11–26 (2014).
Liu, S. et al. TLR2 is a primary receptor for Alzheimer's amyloid b peptide to trigger neuroinflammatory activation. J. Immunol. 188, 1098–1107 (2012).
Stewart, C.R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Lim, J.-E. et al. MyD88 deficiency ameliorates β-amyloidosis in an animal model of Alzheimer's disease. Am. J. Pathol. 179, 1095–1103 (2011).
Lim, J.-E. et al. The effects of MyD88 deficiency on exploratory activity, anxiety, motor coordination, and spatial learning in C57BL/6 and APPswe/PS1dE9 mice. Behav. Brain Res. 227, 36–42 (2012).
Reed-Geaghan, E.G., Savage, J.C., Hise, A.G. & Landreth, G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J. Neurosci. 29, 11982–11992 (2009).
Maezawa, I., Zimin, P.I., Wulff, H. & Jin, L.-W. Amyloid-b protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 286, 3693–3706 (2011).
Wright, A.L. et al. Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer's disease. PLoS ONE 8, e59586 (2013).
Heneka, M.T. et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation 2, 22 (2005).
Sojkova, J. & Resnick, S.M. In vivo human amyloid imaging. Curr. Alzheimer Res. 8, 366–372 (2011).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007).
Cribbs, D.H. et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9, 179 (2012).
Latz, E., Xiao, T.S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 (2008).
Tong, L. et al. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J. Neurosci. 32, 17714–17724 (2012).
Youm, Y.-H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).
Heneka, M.T. et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Cameron, B. et al. Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer's disease. J. Neurosci. 32, 15112–15123 (2012).
Vom Berg, J. et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer's disease-like pathology and cognitive decline. Nat. Med. 18, 1812–1819 (2012).
Terwel, D. et al. Critical role of astroglial apolipoprotein E and liver X receptor-a expression for microglial Aβ phagocytosis. J. Neurosci. 31, 7049–7059 (2011).
Vodovotz, Y. et al. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J. Exp. Med. 184, 1425–1433 (1996).
Hoshino, T. et al. Suppression of Alzheimer's disease-related phenotypes by expression of heat shock protein 70 in mice. J. Neurosci. 31, 5225–5234 (2011).
Paolicelli, R.C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Yirmiya, R. & Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213 (2011).
Lynch, M.A. Neuroinflammatory changes negatively impact on LTP: A focus on IL-1β. Brain Res. doi:10.1016/j.brainres.2014.08.040 (2014).
Pickering, M., Cumiskey, D. & O'Connor, J.J. Actions of TNF-a on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 90, 663–670 (2005).
Cumiskey, D., Pickering, M. & O'Connor, J.J. Interleukin-18 mediated inhibition of LTP in the rat dentate gyrus is attenuated in the presence of mGluR antagonists. Neurosci. Lett. 412, 206–210 (2007).
Wang, Q., Rowan, M.J. & Anwyl, R. β-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J. Neurosci. 24, 6049–6056 (2004).
Bachstetter, A.D. et al. Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer's disease-related pathology. J. Neurosci. 32, 10201–10210 (2012).
Lee, M. et al. Acidic fibroblast growth factor (FGF) potentiates glial-mediated neurotoxicity by activating FGFR2 IIIb protein. J. Biol. Chem. 286, 41230–41245 (2011).
Cho, S.-H. et al. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 286, 32713–32722 (2011).
Neniskyte, U., Neher, J.J. & Brown, G.C. Neuronal death induced by nanomolar amyloid b is mediated by primary phagocytosis of neurons by microglia. J. Biol. Chem. 286, 39904–39913 (2011).
Kitazawa, M., Oddo, S., Yamasaki, T.R., Green, K.N. & LaFerla, F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 25, 8843–8853 (2005).
Lee, C.Y. & Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 (2010).
Bhaskar, K. et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31 (2010).
Sy, M. et al. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am. J. Pathol. 178, 2811–2822 (2011).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Gorlovoy, P., Larionov, S., Pham, T.T.H. & Neumann, H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 23, 2502–2513 (2009).
Ghosh, S. et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J. Neurosci. 33, 5053–5064 (2013).
Heneka, M.T. et al. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 107, 6058–6063 (2010).
Kummer, M.P. et al. Mrp14 deficiency ameliorates amyloid b burden by increasing microglial phagocytosis and modulation of amyloid precursor protein processing. J. Neurosci. 32, 17824–17829 (2012).
Grommes, C. et al. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J. Neuroimmune Pharmacol. 3, 130–140 (2008).
Zhu, Y. et al. CD45 deficiency drives amyloid-β peptide oligomers and neuronal loss in Alzheimer's disease mice. J. Neurosci. 31, 1355–1365 (2011).
Yuyama, K., Sun, H., Mitsutake, S. & Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 287, 10977–10989 (2012).
Hollingworth, P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 43, 429–435 (2011).
Liang, Y. & Tedder, T.F. Identification of a CD20-, FceRIβ-, and HTm4-related gene family: sixteen new MS4A family members expressed in human and mouse. Genomics 72, 119–127 (2001).
Lajaunias, F., Dayer, J.-M. & Chizzolini, C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 35, 243–251 (2005).
Bradshaw, E.M. et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 16, 848–850 (2013).
Griciuc, A. et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid b. Neuron 78, 631–643 (2013).
Melchior, B. et al. Dual induction of TREM2 and tolerance-related transcript, Tmem176b, in amyloid transgenic mice: implications for vaccine-based therapies for Alzheimer's disease. ASN Neuro 2, e00037 (2010).
Frank, S. et al. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia 56, 1438–1447 (2008).
Hamerman, J.A. et al. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J. Immunol. 177, 2051–2055 (2006).
Bouchon, A., Hernández-Munain, C., Cella, M. & Colonna, M.A. DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 194, 1111–1122 (2001).
Takata, K. et al. Galantamine-induced amyloid-β clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J. Biol. Chem. 285, 40180–40191 (2010).
Mandrekar-Colucci, S., Karlo, J.C. & Landreth, G.E. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-g-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. J. Neurosci. 32, 10117–10128 (2012).
Yamanaka, M. et al. PPARg/RXRa-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32, 17321–17331 (2012).
Adolfsson, R., Gottfries, C.G., Roos, B.E. & Winblad, B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br. J. Psychiatry 135, 216–223 (1979).
Leissring, M.A. et al. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 (2003).
Tamboli, I.Y. et al. Statins promote the degradation of extracellular amyloid β-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 285, 37405–37414 (2010).
Kumar, S. et al. Phosphorylation of amyloid-β peptide at serine 8 attenuates its clearance via insulin-degrading and angiotensin-converting enzymes. J. Biol. Chem. 287, 8641–8651 (2012).
Sheng, J.G. et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid b peptide in APPswe transgenic mice. Neurobiol. Dis. 14, 133–145 (2003).
Weberpals, M. et al. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J. Neurosci. 29, 14177–14184 (2009).
Lemstra, A.W. et al. Microglia activation in sepsis: a case-control study. J. Neuroinflammation 4, 4 (2007).
Holmes, C. et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73, 768–774 (2009).
Iwashyna, T.J., Ely, E.W., Smith, D.M. & Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. J. Am. Med. Assoc. 304, 1787–1794 (2010).
Semmler, A. et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry 84, 62–69 (2013).
Kamer, A.R. et al. TNF-a and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J. Neuroimmunol. 216, 92–97 (2009).
Kamer, A.R. et al. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimers Dement. 4, 242–250 (2008).
Lumeng, C.N., Bodzin, J.L. & Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Uryu, K. et al. Repetitive mild brain trauma accelerates Aβ deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 22, 446–454 (2002).
Stalder, M. et al. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am. J. Pathol. 154, 1673–1684 (1999).
Simard, A.R., Soulet, D., Gowing, G., Julien, J.-P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 (2006).
Acknowledgements
Supported by the Deutsche Forschungsgemeinschaft (Excellence Cluster ImmunoSensation and KFO177 to E.L. and M.T.H.) and by the US National Institutes of Health (1R01HL112661 to E.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Heneka, M., Golenbock, D. & Latz, E. Innate immunity in Alzheimer's disease. Nat Immunol 16, 229–236 (2015). https://doi.org/10.1038/ni.3102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3102
This article is cited by
-
The neuroprotective N-terminal amyloid-β core hexapeptide reverses reactive gliosis and gliotoxicity in Alzheimer’s disease pathology models
Journal of Neuroinflammation (2023)
-
Investigation of early molecular alterations in tauopathy with generative adversarial networks
Scientific Reports (2023)
-
Elevated Levels of Naturally-Occurring Autoantibodies Against the Extracellular Domain of p75NTR Aggravate the Pathology of Alzheimer’s Disease
Neuroscience Bulletin (2023)
-
Possible role of diabetes and related mechanisms in COVID-19-induced cognitive impairment
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Tissue-resident immune cells in the pathogenesis of multiple sclerosis
Inflammation Research (2023)