Abstract
Immune responses need to be tightly controlled to avoid excessive inflammation and prevent unwanted host damage. Here we report that germinal center kinase MST4 responded dynamically to bacterial infection and acted as a negative regulator of inflammation. We found that MST4 directly interacted with and phosphorylated the adaptor TRAF6 to prevent its oligomerization and autoubiquitination. Accordingly, MST4 did not inhibit lipopolysaccharide-induced cytokine production in Traf6−/− embryonic fibroblasts transfected to express a mutant form of TRAF6 that cannot be phosphorylated at positions 463 and 486 (with substitution of alanine for threonine at those positions). Upon developing septic shock, mice in which MST4 was knocked down showed exacerbated inflammation and reduced survival, whereas heterozygous deletion of Traf6 (Traf6+/−) alleviated such deleterious effects. Our findings reveal a mechanism by which TRAF6 is regulated and highlight a role for MST4 in limiting inflammatory responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 (2004).
Ye, H. et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418, 443–447 (2002).
Muzio, M., Ni, J., Feng, P. & Dixit, V.M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278, 1612–1615 (1997).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424, 793–796 (2003).
Boone, D.L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Shembade, N., Ma, A. & Harhaj, E.W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Yuk, J.M. et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat. Immunol. 12, 742–751 (2011).
Wu, Y. et al. HSP27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cell. Signal. 21, 143–150 (2009).
Zhang, X., Zhang, J., Zhang, L., van Dam, H. & ten Dijke, P. UBE2O negatively regulates TRAF6-mediated NF-κB activation by inhibiting TRAF6 polyubiquitination. Cell Res. 23, 366–377 (2013).
Ceccarelli, D.F. et al. CCM3/PDCD10 heterodimerizes with germinal center kinase III (GCKIII) proteins using a mechanism analogous to CCM3 homodimerization. J. Biol. Chem. 286, 25056–25064 (2011).
Zhang, M. et al. Structural mechanism of CCM3 heterodimerization with GCKIII kinases. Structure 21, 680–688 (2013).
Qian, Z., Lin, C., Espinosa, R., LeBeau, M. & Rosner, M.R. Cloning and characterization of MST4, a novel Ste20-like kinase. J. Biol. Chem. 276, 22439–22445 (2001).
Lin, J.L. et al. MST4, a new Ste20-related kinase that mediates cell growth and transformation via modulating ERK pathway. Oncogene 20, 6559–6569 (2001).
Liu, G. et al. Structure of MST2 SARAH domain provides insights into its interaction with RAPL. J. Struct. Biol. 185, 366–374 (2014).
Jiao, S. et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25, 166–180 (2014).
Chen, C. et al. Striatins contain a noncanonical coiled coil that binds PP2A A to form a 2:2 heterotetrameric core of striatin-interacting phosphatase and kinase (STRIPAK) complex. J. Biol. Chem. 289, 9651–9661 (2014).
Ma, X. et al. PDCD10 interacts with Ste20-related kinase MST4 to promote cell growth and transformation via modulation of the ERK pathway. Mol. Biol. Cell 18, 1965–1978 (2007).
ten Klooster, J.P. et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 16, 551–562 (2009).
Kean, M.J. et al. Structure-function analysis of core STRIPAK proteins: a signaling complex implicated in Golgi polarization. J. Biol. Chem. 286, 25065–25075 (2011).
Shi, Z. et al. Structure of the MST4 in complex with MO25 provides insights into its activation mechanism. Structure 21, 449–461 (2013).
Xu, X., Wang, X., Zhang, Y., Wang, D.C. & Ding, J. Structural basis for the unique heterodimeric assembly between cerebral cavernous malformation 3 and germinal center kinase III. Structure 21, 1059–1066 (2013).
DeForge, L.E. & Remick, D.G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem. Biophys. Res. Commun. 174, 18–24 (1991).
Tang, E.D., Wang, C.Y., Xiong, Y. & Guan, K.L. A role for NF-κB essential modifier/IκB kinase-γ (NEMO/IKKγ) ubiquitination in the activation of the IκB kinase complex by tumor necrosis factor-α. J. Biol. Chem. 278, 37297–37305 (2003).
Zhou, H. et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 (2004).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Ea, C.K., Sun, L., Inoue, J. & Chen, Z.J. TIFA activates IκB kinase (IKK) by promoting oligomerization and ubiquitination of TRAF6. Proc. Natl. Acad. Sci. USA 101, 15318–15323 (2004).
Ni, C.Z. et al. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. USA 97, 10395–10399 (2000).
Janssens, S. & Beyaert, R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 27, 474–482 (2002).
Wertz, I.E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Allen, I.C. et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34, 854–865 (2011).
Schneider, M. et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 13, 823–831 (2012).
Massoumi, R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem. Sci. 35, 392–399 (2010).
Hymowitz, S.G. & Wertz, I.E. A20: from ubiquitin editing to tumour suppression. Nat. Rev. Cancer 10, 332–341 (2010).
Rosas, M. et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344, 645–648 (2014).
Madsen, C.D. et al. STRIPAK components determine mode of cancer cell migration and metastasis. Nat. Cell Biol. 17, 68–80 (2015).
Starczynowski, D.T. et al. TRAF6 is an amplified oncogene bridging the RAS and NF-κB pathways in human lung cancer. J. Clin. Invest. 121, 4095–4105 (2011).
Mu, Y. et al. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat. Comm. 2, 330 (2011).
Liang, J. et al. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κB signaling. J. Exp. Med. 207, 2959–2973 (2010).
Yang, W.L. et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325, 1134–1138 (2009).
Gudey, S.K. et al. TRAF6 stimulates the tumor-promoting effects of TGFβ type I receptor through polyubiquitination and activation of presenilin 1. Sci. Signal. 7, ra2 (2014).
Ge, Q. et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. USA 101, 8676–8681 (2004).
Williams, J.M. et al. The role of the Wnt family of secreted proteins in rat oval “stem” cell-based liver regeneration: Wnt1 drives differentiation. Am. J. Pathol. 176, 2732–2742 (2010).
Wang, R., Xu, Y.J., Liu, X.S., Zeng, D.X. & Xiang, M. Knockdown of connective tissue growth factor by plasmid-based short hairpin RNA prevented pulmonary vascular remodeling in cigarette smoke-exposed rats. Arch. Biochem. Biophys. 508, 93–100 (2011).
Liu, Y. et al. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J. Cell Biol. 201, 863–873 (2013).
Timchenko, L.T. et al. Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein β in old liver. J. Biol. Chem. 281, 32806–32819 (2006).
Austyn, J.M. & Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11, 805–815 (1981).
Deng, L. et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 (2000).
Lou, X. et al. Negative feedback regulation of NF-κB action by CITED2 in the nucleus. J. Immunol. 186, 539–548 (2011).
Acknowledgements
We thank H. Ji (Shanghai Institute of Biochemistry and Cell Biology) for the pLKO.1-puro backbone; and D. Li, J. Zhou, G. Pei, B. Sun, L. Li, A. Lin and M. Lei for support. Supported by the 973 program of the Ministry of Science and Technology of China (2012CB910204 and 2012CB910200), the National Natural Science Foundation of China (31270808, 31300734, 31470736, 31470868, 31030021 and 81161120542), the Science and Technology Commission of Shanghai Municipality (11JC14140000 and 13ZR1446400), and the “Cross and cooperation in science and technology innovation team” project of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
S.J., Zhe.Z. and Chuan.L. designed and carried out most of the experiments and analyzed the data; M.H. and C.C.L.W. performed mass spectrometry analysis; Z.S. did the structural modeling; Y.W. and M.C. provided purified proteins. X.S., H.L., Chun.L. and W.W. contributed to data analysis; Zha.Z., Y.Z., Z.J., H.W. and C.W. contributed to experimental design; S.J., X.S. and Zha.Z. wrote the manuscript; and Zha.Z. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
Zha.Z. and S.J. have filed a patent (201410705068.X) related to this work, which covers the potential application of MST4 as a biomarker for certain infectious diseases, and possible therapeutics relevant to the MST4 function in macrophages.
Integrated supplementary information
Supplementary Figure 1 Fluctuation of MST4 expression in response to LPS stimulation or bacterial infection.
(a,b) Scatter plot for MST4 mRNA levels in sepsis data sets GSE54514 (a) and GSE26378 (b). (c) Relative mRNA levels of MST4, TNF and IL6 upon LPS challenge at different time points. (d) Transcription of Mst4 in mouse AVECs, AECs, LFs, and AMs upon SL1344 infection. (e) Immunoblot (IB throughout) analysis for MST4 (arrowhead along right margin throughout) in cells transfected with Flag-MST4 and MST4-specific shRNA. (f) Immunoblot of mouse tissues with MST4 antibody. Mouse tissues were prepared and quantified using the bicinchoninic acid assay (50 mg protein loaded per sample), analyzed with polyclonal anti-MST4 antibody. Data are representative of at least two experiments.
Supplementary Figure 2 MST4 negatively regulates TLR signaling.
(a) Relative mRNA levels of TNF and I L6 in THP-1 cells expressing MST4 upon LPS challenge. (b) Production of TNF and IL-6 in THP-1 cells expressing MST4 upon LPS challenge. (c) Secretion of cytokines in shMST4 THP-1 cells in response to LPS challenge. (d) Il1b mRNA levels in PEMs expressing MST4 upon SL1344 infection. (e) Il1b mRNA levels in shMST4 PEMs challenged with different concentrations of LPS. (f) Transcription of cytokines in shMST4 THP-1 cells challenged with different concentrations of LPS. (g) Production of TNF and IL-6 in shMST4 THP-1 after LPS tolerance. (h, i) Il6 mRNA levels in control and MST4-knockdown MEFs (h) and PEMs (i) upon acute LPS challenge. *, P<0.05; **, P<0.01; ***, P<0.001 compared with control group (unpaired t-test). Data are representative of at least two experiments (mean and s.d.). EV, empty vector (same below); Scr, control shRNA.
Supplementary Figure 3 MST4 regulates TLR signaling dependent on its kinase activity.
(a) Immunoblot analysis of total IκBα in THP-1 cells after transfection with MST4 and its mutants upon LPS treatment. (b) Immunoblot of MST4 in shMST4 MEFs after transfection with Flag-MST4 and its mutants. (c) The interaction between MST4 and MO25 in the lung and bone marrow of mice treated with LPS for the indicated time period. (d) LPS-induced IL-6 production after transfection with MST4 and MO25 in THP-1 cells. **, P<0.01; ***, P<0.001 compared with control group (unpaired t-test). Data are representative of at least two experiments (means and s.d. in d).
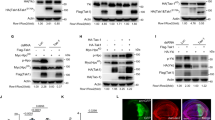
Supplementary Figure 4 MST4 directly interacts with TRAF6.
(a) Luciferase activity detected in HEK 293T cells transfected with a NF-κB luciferase reporter and indicated plasmids. (b) NF-κB activation induced by TRAF6 after transfection with MST4 and MO25. (c) Exogenous IP for MST4 and TRAF6. (d) Co-localization of MST4 and TRAF6 in THP-1 cells upon LPS stimulation. (e) Schematic illustration of the domain organization of MST4 and TRAF6. (f, g) Co-immunoprecipitation (IP throughout) mapping of specific domains responsible for MST4 interaction with TRAF6. (h) Pulldown assay for testing a direct interaction between MST4 and TRAF6-C. *, P<0.05; **, P<0.01; ***, P<0.001 (unpaired t-test). Data are representative of at least two experiments. TRAF6-C, TRAF-C domain of TRAF6.
Supplementary Figure 5 MST4 inhibits TRAF6 autoubiquitination in a manner dependent on its kinase activity.
(a) Immunoblot of TRAF6 ubiquitination in THP-1 cells upon LPS challenge. (b) Ubiquitination of TRAF6 after transfection with MST4 or its constitutively active mutant (TE) or kinase dead mutant (KR). (c) Ubiquitination of TRAF6 in HEK 293T cells overexpressing MST4 and MO25. (d) Ubiquitination of NEMO in cells overexpressing MST4 and its mutants. Data are representative of at least two experiments.
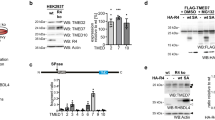
Supplementary Figure 6 MST4 directly phosphorylates the TRAF-C domain of TRAF6.
(a) Ubiquitination and Ser/Thr phosphorylation of TRAF6 in THP-1 cells transfected with shMST4 upon LPS challenge. (b) Domain mapping of TRAF6 phosphorylation by MST4. (c) Mass spectrometric analysis of phosphorylation sites of TRAF6 by MST4. (d) In vitro kinase assay of wild-type MST4 using wild-type TRAF-C domain of TRAF6 (TRAF6-C (WT)) or its mutant with threonines 463 and 486 substituted with alanines (TRAF6-C (2A)) as substrate.
Supplementary Figure 7 MST4 interferes with the assembly of TRAF6-related signaling complex.
Gel-filtration assays for signaling complexes containing endogenous TRAF6 in TNFα-stimulated 293T cells. Fractions of gel-filtration were immunoblotted with antibody against TRAF6. Data are representative of at least two experiments.
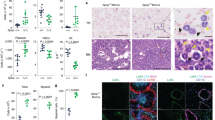
Supplementary Figure 8 In vivo knockdown of MST4 in mice upon septic shock.
(a,b) Transcription of Mst4 (a) and Il6 (b) in the different organs from MST4-KD mice upon intraperitoneal injection with LPS. (c) Relative mRNA change of Mst4 and Il6 in MST4-KD mice compared with mock mice. (d) Plasma cytokine expression in mice treated with LPS (n=5). (e) Flow cytometry of F4/80+ macrophages isolated from spleens 2 days after clodronate liposome treatment. *, P<0.05 (unpaired t-test). Data are representative of at least two experiments (means and s.d. in d).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2942 kb)
Rights and permissions
About this article
Cite this article
Jiao, S., Zhang, Z., Li, C. et al. The kinase MST4 limits inflammatory responses through direct phosphorylation of the adaptor TRAF6. Nat Immunol 16, 246–257 (2015). https://doi.org/10.1038/ni.3097
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3097
This article is cited by
-
Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice
Nature Communications (2024)
-
MST4 kinase regulates immune thrombocytopenia by phosphorylating STAT1-mediated M1 polarization of macrophages
Cellular & Molecular Immunology (2023)
-
The protease calpain2a limits innate immunity by targeting TRAF6 in teleost fish
Communications Biology (2023)
-
SUN1/2 controls macrophage polarization via modulating nuclear size and stiffness
Nature Communications (2023)
-
FKBP51 plays an essential role in Akt ubiquitination that requires Hsp90 and PHLPP
Cell Death & Disease (2023)