Abstract
The recognized diversity of innate lymphoid cells (ILCs) is rapidly expanding. Three ILC classes have emerged, ILC1, ILC2 and ILC3, with ILC1 and ILC3 including several subsets. The classification of some subsets is unclear, and it remains controversial whether natural killer (NK) cells and ILC1 cells are distinct cell types. To address these issues, we analyzed gene expression in ILCs and NK cells from mouse small intestine, spleen and liver, as part of the Immunological Genome Project. The results showed unique gene-expression patterns for some ILCs and overlapping patterns for ILC1 cells and NK cells, whereas other ILC subsets remained indistinguishable. We identified a transcriptional program shared by small intestine ILCs and a core ILC signature. We revealed and discuss transcripts that suggest previously unknown functions and developmental paths for ILCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Heng, T.S. & Painter, M.W. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Diefenbach, A., Colonna, M. & Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41, 354–365 (2014).
McKenzie, A.N., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014).
Klose, C.S. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Sawa, S. et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 330, 665–669 (2010).
Lee, J.S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–151 (2011).
Klose, C.S. et al. A T-bet gradient controls the fate and function of CCR6-Rorγt+ innate lymphoid cells. Nature 494, 262–265 (2013).
Sciumé, G. et al. Distinct requirements for T-bet in gut innate lymphoid cells. J. Exp. Med. 209, 2331–2338 (2012).
Rankin, L.C. et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14, 389–395 (2013).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Constantinides, M.G., McDonald, B.D., Verhoef, P.A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Yu, J., Freud, A.G. & Caligiuri, M.A. Location and cellular stages of natural killer cell development. Trends Immunol. 34, 573–582 (2013).
Gordon, S.M. et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 36, 55–67 (2012).
Reynders, A. et al. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt– lymphoid cells. EMBO J. 30, 2934–2947 (2011).
Gasteiger, G., Hemmers, S., Bos, B.D., Sun, J.C. & Rudensky, A.Y. IL-2-dependent adaptive control of NK cell homeostasis. J. Exp. Med. 210, 1179–1187 (2013).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38, 769–781 (2013).
Turner, J.E. et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 210, 2951–2965 (2013).
Mjösberg, J. et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 37, 649–659 (2012).
Yagi, R. et al. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity 40, 378–388 (2014).
Evans, R.M. & Mangelsdorf, D.J. Nuclear receptors, RXR, and the Big Bang. Cell 157, 255–266 (2014).
Spencer, S.P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Molofsky, A.B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Mohebiany, A.N., Harroch, S. & Bouyain, S. in Cell Adhesion Molecules: Implications in Neurological Diseases, Advances in Neurobiology Vol. 8 (eds. Berezin, V. & Walmod, P.S.) Chapter 8 (Springer Science+Business Media, 2014).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal tolerance. Science 343, 1249288 (2014).
Levitt, L.J. et al. Production of granulocyte/macrophage-colony stimulating factor by human natural killer cells. Modulation by the p75 subunit of the interleukin 2 and by the CD2 receptor. J. Clin. Invest. 88, 67–75 (1991).
van de Pavert, S.A. et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508, 123–127 (2014).
Kiss, E.A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Gascoyne, D.M. et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat. Immunol. 10, 1118–1124 (2009).
Kamizono, S. et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J. Exp. Med. 206, 2977–2986 (2009).
Seillet, C. et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J. Exp. Med. 211, 1733–1740 (2014).
Geiger, T.L. et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J. Exp. Med. 211, 1723–1731 (2014).
Muller, P.A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Bando, J.K., Liang, H.E. & Locksley, R.M. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat. Immunol. http://www.nature.com/ni/journal/vaop/ncurrent/full/ni.3057.html (2014).
Veiga-Fernandes, H. et al. Tyrosine receptor RET is a key regulator of Peyer's patch organogenesis. Nature 446, 547–551 (2007).
Ramirez, K. et al. Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity 36, 921–932 (2012).
Carpenter, S., Ricci, E.P., Mercier, B.C., Moore, M.J. & Fitzgerald, K.A. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol. 14, 361–376 (2014).
Kumanogoh, A. & Kikutani, H. Immunological functions of the neuropillins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 13, 802–814 (2013).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722 (2012).
Weiss, J.M. et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742 (2012).
Shen, W. et al. Adaptive immunity to murine skin commensals. Proc. Natl. Acad. Sci. USA 111, E2977–E2986 (2014).
Daussy, C. et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 211, 563–577 (2014).
Sojka, D.K. et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife 3, e01659 (2014).
Ye, S.K. et al. Induction of germline transcription in the Tcrγ locus by Stat5: implications for accessibility control by the IL-7 receptor. Immunity 11, 213–223 (1999).
Zhao, H., Nguyen, H. & Kang, J. Interleukin 15 controls the generation of restricted T cell receptor repertoire of intraepithelial lymphocytes. Nat. Immunol. 6, 1263–1271 (2005).
Allan, D.S. et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol. http://www.nature.com/mi/journal/vaop/ncurrent/full/mi201471a.html (2014).
Yu, X. et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell progenitor. Elife 10, e04406 (2014).
Satoh-Takayama, N. et al. The chemokine receptor CXCR6 controls the functional topograophy of interluekin-22 producing intestinal innate lymphoid cells. Immunity 41, 776–788 (2014).
Bezman, N.A. et al. ImmGen report: molecular definition of natural killer cell identity and activation. Nat. Immunol. 13, 1000–1009 (2012).
Peng, H. et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 123, 1444–1456 (2013).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Acknowledgements
We thank our colleagues in the ImmGen consortium, especially C. Benoist and L. Lanier, for input and discussion; the core ImmGen team, K. Rothamel and A. Rhodes, for contributions and technical assistance; M. Artyomov, G. Krishnan and J. Siegel for computational assistance; D. Sojka for discussion; E. Lantelme and D. Brinja for sorting assistance; P. Wang for microscopy assistance; and eBioscience and Affymetrix for support of the ImmGen Project. Supported by the US National Institutes of Health (R24AI072073 to the ImmGen Consortium; 1U01AI095542, R01DE021255 and R21CA16719 to the Colonna laboratory; MSTP T32 GM07200 to M.L.R.; and Infectious Disease Training Grant T32 AI 7172-34 to V.S.C.).
Author information
Authors and Affiliations
Consortia
Contributions
M.L.R. analyzed data; A.F., M.L.R., J.S.L. and Y.W. sorted cell subsets; M.L.R., A.F. and V.S.C. performed follow-up experiments and analyzed data; S.G. maintained mice; S.K.D. provided critical reagents; M.L.R., A.F., S.G. and M.C. designed studies; M.L.R. and M.C. wrote the paper; and the ImmGen Consortium contributed to the experimental design and data collection.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
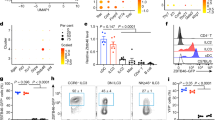
Supplementary Figure 1 Characteristics of sorted ILC and NK cell populations.
(a) Gating strategy for siLP ILC1, NK, NKp46+ ILC3, and NKp46− LTi-like ILC3 subsets after gating on live CD45+ and CD3−CD19− cells in C57BL/6 mice or RorγteGFP/+ reporter mice. ILCs are gated on intracellularly stained Rorγt+ cells (left) or RorγteGFP+ cells (right). For microarray analysis, the gating strategy as shown on the right was used. (b,c) Histograms of NK1.1 (b) and CD45 (c) expression from sorted siLP subsets used in array generation (n = 5 pooled mice each). (d) Two-dimensional views of PCA. Data are representative of at least ten experiments (a) or five independent experiments (b,c).
Supplementary Figure 2 Validation of ILC core signature.
(a) Extracellular and intracellular staining of TCRδ on (or in) ILCs and NK cells, continued from Figure 6. Numbers in outlined areas indicate percent TCRδ+ cells (outline colors (red) match those in keys above plots). (b) Representative flow plots of percentage of CXCR6+ cells from CXCR6eGFP/+ reporter mice. siLP NKp46− LTi-like ILC3, siLP ILC1, siLP NK, and siLP NKp46+ ILC3 percentages were analyzed after fixation of cells. Color-coded gates demonstrate percentage of indicated subsets that are CXCR6+ (n = three or four mice per genotype per tissue). Data are representative of two independent experiments (a) or three independent experiments (b).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 1153 kb)
Supplementary Table 1
Unique transcripts of individual ILC subsets (XLSX 50 kb)
Supplementary Table 2
Shared transcripts between siILC subsets (XLSX 73 kb)
Supplementary Table 3
Transcripts differentially expressed among ILC3 subsets (XLSX 60 kb)
Supplementary Table 4
Transcripts differentially expressed between NK cells and ILC1 cells in a single tissue (XLSX 84 kb)
Supplementary Table 5
Transcripts differentially expressed between NK cells and ILC1 cells in two tissues (XLSX 48 kb)
Supplementary Table 6
Core ILC1 and NK cell signatures (XLSX 54 kb)
Source data
Rights and permissions
About this article
Cite this article
Robinette, M., Fuchs, A., Cortez, V. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol 16, 306–317 (2015). https://doi.org/10.1038/ni.3094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3094
This article is cited by
-
Cooperation of ILC2s and TH2 cells in the expulsion of intestinal helminth parasites
Nature Reviews Immunology (2024)
-
Nutrition impact on ILC3 maintenance and function centers on a cell-intrinsic CD71–iron axis
Nature Immunology (2023)
-
Gene expression profiling in NOD mice reveals that B cells are highly educated by the pancreatic environment during autoimmune diabetes
Diabetologia (2023)
-
Neuropilin-1 flags lung-resident type 2 innate lymphoid cells
Nature Immunology (2022)
-
Neuropilin-1 mediates lung tissue-specific control of ILC2 function in type 2 immunity
Nature Immunology (2022)