Abstract
The quality of the adaptive immune response depends on the differentiation of distinct CD4+ helper T cell subsets, and the magnitude of an immune response is controlled by CD4+Foxp3+ regulatory T cells (Treg cells). However, how a tissue- and cell type–specific suppressor program of Treg cells is mechanistically orchestrated has remained largely unexplored. Through the use of Treg cell–specific gene targeting, we found that the suppression of allergic immune responses in the lungs mediated by T helper type 2 (TH2) cells was dependent on the activity of the protein kinase CK2. Genetic ablation of the β-subunit of CK2 specifically in Treg cells resulted in the proliferation of a hitherto-unexplored ILT3+ Treg cell subpopulation that was unable to control the maturation of IRF4+PD-L2+ dendritic cells required for the development of TH2 responses in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Accession codes
References
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Brunkow, M.E. et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27, 68–73 (2001).
Wildin, R.S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 (2001).
Kim, J.M., Rasmussen, J.P. & Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Tang, Q. & Bluestone, J.A. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9, 239–244 (2008).
Kyttaris, V.C. Kinase inhibitors: a new class of antirheumatic drugs. Drug. Des. Devel. Ther. 6, 245–250 (2012).
Dominguez, I., Sonenshein, G.E. & Seldin, D.C. CK2 and its role in Wnt and NF-kB signaling: linking development and cancer. Cell. Mol. Life Sci. 66, 1850–1857 (2009).
Trembley, J.H., Wang, G., Unger, G., Slaton, J. & Ahmed, K. Protein kinase CK2 in health and disease. Cell. Mol. Life Sci. 66, 1858–1867 (2009).
Seldin, D.C. et al. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol. Cell. Biochem. 274, 63–67 (2005).
Buchou, T. et al. Disruption of the regulatory β subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 23, 908–915 (2003).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Kashiwada, M. et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc. Natl. Acad. Sci. USA 107, 821–826 (2010).
Ponce, D.P. et al. CK2 functionally interacts with AKT/PKB to promote the β-catenin-dependent expression of survivin and enhance cell survival. Mol. Cell. Biochem. 356, 127–132 (2011).
Song, D.H. CK2 Phosphorylation of the armadillo repeat region of β-catenin potentiates Wnt signaling. J. Biol. Chem. 278, 24018–24025 (2003).
Song, D.H. Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J. Biol. Chem. 275, 23790–23797 (2000).
Ponce, D.P. et al. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of β-catenin transcriptional activity. J. Cell. Physiol. 226, 1953–1959 (2011).
Larriba, M. et al. Vitamin D is a multilevel repressor of Wnt/β-catenin signaling in cancer cells. Cancers 5, 1242–1260 (2013).
Rochat, M.K. et al. Maternal vitamin D intake during pregnancy increases gene expression of ILT3 and ILT4 in cord blood. Clin. Exp. Allergy 40, 786–794 (2010).
Lu-Kuo, J.M., Joyal, D.M., Austen, K.F. & Katz, H.R. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization. J. Biol. Chem. 274, 5791–5796 (1999).
Lorenz, U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol. Rev. 228, 342–359 (2009).
Moran, A.E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Sekiya, T. et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 14, 230–237 (2013).
Gao, Y. et al. Control of T Helper 2 Responses by Transcription Factor IRF4-Dependent Dendritic Cells. Immunity 39, 722–732 (2013).
Williams, J.W. et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 4, 2990 (2013).
Sakaguchi, S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Xystrakis, E., Urry, Z. & Hawrylowicz, C.M. Regulatory T cell therapy as individualized medicine for asthma and allergy. Curr. Opin. Allergy Clin. Immunol. 7, 535–541 (2007).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Amsen, D. et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27, 89–99 (2007).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458, 351–356 (2009).
Josefowicz, S.Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Gottschalk, R.A., Corse, E. & Allison, J.P. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J. Immunol. 188, 976–980 (2012).
Hori, S. Stability of regulatory T-cell lineage. Adv. Immunol. 112, 1–24 (2011).
Meggio, F. & Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17, 349–368 (2003).
Iype, T., Sankarshanan, M., Mauldin, I.S., Mullins, D.W. & Lorenz, U. The protein tyrosine phosphatase SHP-1 modulates the suppressive activity of regulatory T cells. J. Immunol. 185, 6115–6127 (2010).
Bottema, R.W.B. et al. Interaction of T-cell and antigen presenting cell co-stimulatory genes in childhood IgE. Eur. Respir. J. 35, 54–63 (2009).
Cozza, G., Pinna, L.A. & Moro, S. Protein kinase CK2 inhibitors: a patent review. Expert Opin. Ther. Pat. 22, 1081–1097 (2012).
Lahl, K. et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204, 57–63 (2007).
Mittrücker, H.W. et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 275, 540–543 (1997).
Tuettenberg, A. et al. Induction of strong and persistent MelanA/MART-1-specific immune responses by adjuvant dendritic cell-based vaccination of stage II melanoma patients. Int. J. Cancer 118, 2617–2627 (2006).
Muth, S., Schütze, K., Schild, H. & Probst, H.-C. Release of dendritic cells from cognate CD4+ T-cell recognition results in impaired peripheral tolerance and fatal cytotoxic T-cell mediated autoimmunity. Proc. Natl. Acad. Sci. USA 109, 9059–9064 (2012).
Ruedl, C., Rieser, C., Böck, G., Wick, G. & Wolf, H. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer's patches. Eur. J. Immunol. 26, 1801–1806 (1996).
Bopp, T. et al. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 181–187 (2005).
Jonuleit, H. et al. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196, 255–260 (2002).
Dehzad, N. et al. Regulatory T cells more effectively suppress Th1-induced airway inflammation compared with Th2. J. Immunol. 186, 2238–2244 (2011).
Diks, S.H. et al. Kinome profiling for studying lipopolysaccharide signal transduction in human peripheral blood mononuclear cells. J. Biol. Chem. 279, 49206–49213 (2004).
Staudt, V. et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33, 192–202 (2010).
Powrie, F., Leach, M.W., Mauze, S., Caddle, L.B. & Coffman, R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5, 1461–1471 (1993).
Webster, K.E. et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 206, 751–760 (2009).
Hartmann, W., Haben, I., Fleischer, B. & Breloer, M. Pathogenic nematodes suppress humoral responses to third-party antigens in vivo by IL-10-mediated interference with Th cell function. J. Immunol. 187, 4088–4099 (2011).
Acknowledgements
We thank S. Sakaguchi (Osaka University) for BALB/c Foxp3-IRES-Cre mice; B. Boldyreff (University of Southern Denmark) for Csnk2bfl mice on the C57BL/6 background; T. Sparwasser (TWINCORE) for C57BL/6 DEREG mice; K.A. Hogquist (University of Minnesota) for Nur77GFP mice; M. Lohoff (Philipps University Marburg) for C57BL/6 Irf4−/− mice; H.C. Probst (University Medical Center Mainz) for Ly5.1+ C57BL/6 mice; A. Waisman (University Medical Center Mainz) for CD90.1+ C57BL/6 mice; A. Nikolaev and S. Fischer for technical help, and D. O'Neill for critical reading of the manuscript. Supported by Deutsche Forschungsgemeinschaft (DFG BO 3306/1-1, SCHM 1014/7-1, SCHM 1014/5-1, SFB TR128 projects B4 (T.Bop. and F.Z.), A7 (A.W.) and A9 (H.J.), SFB 1066 projects B1 (E.S.) and B8 (T.Bop.), and BR 3754/2-1 (I.H. and M.B.)), International Graduate School of Immunotherapy (GRK 1043 project C4 (E.S. and T.Bop.)) and Forschungszentrum Immunologie of the University Medical Center of the Johannes Gutenberg-University Mainz (E.S. and T.Bop.).
Author information
Authors and Affiliations
Contributions
A.U. performed and analyzed most experiments; M.K., B.G., M.H., N.G., V.S., N.S., T.Boh., T.-J.B., S.M., H.Y., K.R. and H.-C.P. helped design and perform some experiments; S.Reu. helped to perform and analyze mouse asthma experiments; M.K. conducted RNA-seq experiments; I.B., A.T., S.H., H.J. performed experiments involving human T cells; S.Rei. performed and analyzed adoptive transfer colitis experiments; I.H. and M.B. performed and analyzed nematode infection experiments; F.Z., A.W., A.B., T.Bu., O.F.-C., B.B. and H.S. helped design, analyze and interpret experiments; E.S. and T.Bop. supervised the project, designed experiments and wrote the manuscript; and all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 CK2β deficiency does not alter the number of peripheral Treg cells.
Percentage of Foxp3+ Treg cells among splenic CD4+ T cells in 8-14 week old Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice (n = 6 per group from 2 independent experiments). Data represent mean +/- SD.
Supplementary Figure 2 Schematic representation of experimental setup for asthma experiments.
At day 0 Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice were either sensitized with 20µg Ovalbumin (OVA) emulsified in 2mg aluminium hydroxide (Alum) (OVA in alum) or, as a control group, received PBS via i.p. injection (PBS). On day 15 and 16 following sensitization all animals were challenged with 1% OVA by ultrasonic nebulization via the airways. At day 18 following sensitization animals were sacrificed and analyzed for several experimental readouts as outlined in online methods. (n = 5 mice per group) Data are representative for two independent experiments.
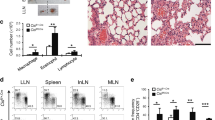
Supplementary Figure 3 Elevated eosinophilia in Csnk2bfl/flFoxp3-Cre mice.
Total cell count of macrophages (MΦ), lymphocytes (lym), neutrophils (neut) and eosinophils (eo), in bronchoalveolar lavage (BAL) of sensitized (OVA in alum) and respective control (PBS) Csnk2bfl/fl as well as Csnk2bfl/flFoxp3-Cre mice after challenge with Ovalbumin (OVA). (n = 5 mice per group). Data represent mean +/- SD and are representative for two independent experiments.
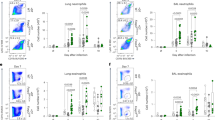
Supplementary Figure 4 Csnk2b-deficient Treg cells show an unaltered ability to home to the lungs.
(a) Flow cytometric analysis of Foxp3 expression in CD4+ T cells from lungs of Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice upon OVA-challenge. Representative flow cytometry plots are shown for each group. (b) Percentage of Foxp3+ Treg cells among CD4+ T cells in lungs of Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice upon OVA-challenge. (n = 5 mice per group). Data represent mean +/- SD and are representative for two independent experiments. Samples were excluded from analysis, if <500 counts of CD4+Foxp3+ cells could be recorded.
Supplementary Figure 5 Unaltered expression of molecules with a presumed role in Treg cell–mediated suppression in the absence of CK2β.
(a) Mean fluorescence intensity (MFI) of the indicated molecules known to be involved in Treg cell-mediated suppression in CD4+Foxp3+ Treg cells of Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice. MFIs are normalized to the average MFI of Treg cells from Csnk2bfl/fl for each experiment (n = 6 mice per group pooled from 2 independent experiments). Data represent mean +/- SD. (b) Percentage of CD4+Foxp3+ Treg cells from Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice expressing the indicated Molecules involved in Treg cell-mediated suppression (CTLA-4 (surface expression and intracellular expression), Granzyme B, CD39 and CD73). Gating was performed according to isotype control staining (n = 6 mice per group pooled from 2 independent experiments). Data represent mean +/- SD. (c) Percentage of CD4+Foxp3+ Treg cells from Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice expressing components of the IL2-receptor complex (CD25, CD122, common γ chain (γc)). (n = 6 mice per group from 2 independent experiments). Data represent mean +/- SD. (d) Naïve CD4+ T cells (Teff) from C57BL/6 mice were labeled with CFSE and stimulated in coculture with unlabeled Csnk2b-deficient (Csnk2b-/-) or Csnk2b-sufficient (Csnk2b+/+) Treg cells in indicated ratios, as outlined in online methods. On day 4, CFSE fluorescence was measured by FACS analyses. Representatives of three independent experiments are shown.
Supplementary Figure 6 ILT3-expressing Treg cells represent a distinct Treg cell subset that is elevated in TH2 cell–mediated inflammation.
(a) Flow cytometric analysis of ILT3 expression on CD4+Foxp3+ Treg cells from different tissues of Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice. Representative flow cytometry plots and percentages of ILT3+ among CD4+Foxp3+ Treg cells in thymus, mesenteric lymph nodes (mLN), spleen and inguinal lymph nodes (iLN) are shown (n = 11 mice per group for thymus and n = 9 per group for mLN, spleen and iLN, combined from 2 independent experiments). Data show mean +/- SD. (b) Flow cytometric analysis of ILT3 expression in CD4+Foxp3+ Treg cells, stimulated for 3 days with plate-bound anti-CD3 and anti-CD28 in the presence of 300ng/ml mrIL-2 and indicated concentration of CK2 inhibitors (DMAT and CX4945). Treg cells were isolated at day 0 by CD25+ magnetic activated cell sorting from spleens of Csnk2b sufficient C57BL/6 mice. Data are representative for three independent experiments. (c) Flow cytometric analysis of ILT3 expression in CD4+Foxp3+ Treg cells from lungs of non-immunized (PBS) or OVA/Alum immunized C57BL/6 mice upon OVA-challenge. Immunization was performed as described in online methods. Representative flow cytometry plots as well as the percentage of ILT3+ among CD4+Foxp3+ Treg cells are shown. (n = 7 for PBS and n = 8 for OVA/alum treated mice pooled from 2 independent experiments). Data represent mean +/- SD. (d) Flow cytometric analysis of ILT3 expression in splenic CD4+Foxp3+ Treg cells from Non-infected and L. sigmodontis infected BALB/c mice at day 76 after infection. Representative FACS plots as well as the percentage of ILT3+ among CD4+Foxp3+ Treg cells are shown. (n = 10 for non-infected and n = 11 for L. sigmodontis infected group pooled from 2 independent experiments). Data represent mean +/- SD. (e) Total cell count in bronchoalveolar lavage fluid (BAL) of mixed bone marrow chimeras (Csnk2bfl/fl + Csnk2bfl/flFoxp3-Cre BMC) (n = 9) as well as from control Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice (n = 3). Csnk2bfl/fl + Csnk2bfl/flFoxp3-Cre BMC were generated as described in online methods. BMCs were analyzed 11 weeks post transfer. Data represent mean +/- SD from one single experiment. (f) Total cell count of eosinophils in bronchoalveolar lavage fluid (BAL) of Csnk2bfl/fl + Csnk2bfl/flFoxp3-Cre BMC as well as from control Csnk2bfl/fl and Csnk2bfl/flFoxp3-Cre mice. Data represent mean +/- SD from one single experiment. (g) Flow cytometric analysis of activated CD62L-CD4+ T cells in the lungs of Csnk2bfl/fl + Csnk2bfl/flFoxp3-Cre BMC, control Csnk2bfl/fl, and Csnk2bfl/flFoxp3-Cre mice. Percentage of CD62L- among CD4+Foxp3- T cells is shown. Data represent mean +/- SD from one single experiment. (h) Flow cytometric analysis of the origin of ILT3+ Treg cells in Csnk2bfl/fl + Csnk2bfl/flFoxp3-Cre BMC. Given is the percentage of ILT3+ among CD4+Foxp3+ Treg cells derived either from CD90.1+ Csnk2b-competent C57BL/6 wildtype (Csnk2bfl/fl BMC) or CD90.2+ Csnk2b-deficient Csnk2bfl/flFoxp3-Cre bone marrow (Csnk2bfl/flFoxp3-Cre BMC). Individual data points form Csnk2bfl/fl BMC or Csnk2bfl/flFoxp3-Cre BMC of respective BMC mice are shown. * = p<0.05; ** = p<0.01; *** = p<0.001. In case of non gausian distribution Mann-Whitney U test was used instead of students t-test.
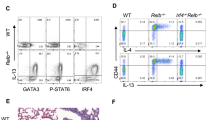
Supplementary Figure 7 Csnk2b-deficient Treg cells are unable to suppress TH2 development.
Flow cytometric analysis of GATA-3 expression in Ly5.1+CD4+ T cells stimulated in absence or presence of either Ly5.2+ Csnk2b-competent (Csnk2b+/+) or Csnk2b-deficient (Csnk2b-/-) Treg cells for 96h as outlined in online methods. Shown are representative FACS-plots of GATA-3+ among CD45.1+CD4+ T cells (a) and the percentage of GATA-3+ among CD45.1+CD4+ T cells (b). Data are combined from 4 independent experiments and represent mean +/- SD. * = p<0.05; ** = p<0.001.
Supplementary Figure 8 ILT3+ Treg cells are able to suppress TH1 responses.
Analysis of IFN-γ-producing CD4+CD45.1+ T cells stimulated in vitro under TH1-polarizing conditions in the absence (Teff) or presence of different numbers of either Csnk2b-competent (Csnk2b+/+) or Csnk2b-deficient (Csnk2b-/-) Treg cells (a) and ILT3- or ILT3+ Treg cells from C57BL/6 wildtype mice (b). For each experiment the amount of IFN-γ producers in absence of Treg cells (Teff) was set to 100% and the percentages of IFN-γ-producing TH1 cells in co-cultures were calculated accordingly. Data show mean +/- SD combined from 4 independent experiments (a) and 3 independent experiments (b). (c) Flow cytometric analysis of T-bet expression in CD4+CD44+CD45.1+ T cells in mesenteric lymph nodes of colitis-bearing mice. Given is the percentage of T-bet+ T cells among CD4+CD44+CD45.1+ T cells (n = 4 for Teff, n = 6 for ILT3- Treg cells and n = 7 for ILT3+ Treg cells from one single experiment). Data represent mean +/- SD. (d) Bodyweight changes upon adoptive transfer of CD4+CD62L+ Teff in absence or presence of ILT3-, ILT3+ Treg cells isolated from C57BL/6 wildtype or Treg cells isolated from either Csnk2bfl/flFoxp3-Cre or Csnk2bfl/fl mice into lymphopenic Rag1-/- mice as described in online methods. (n = 18 for Teff, n = 14 for ILT3- Treg cells and ILT3+ Treg cells, n = 5 for Csnk2b-/- Treg cells and n = 7 for Csnk2b+/+ Treg cells from 2 independent experiments. * = p =0.0212; ** = p = 0.0181; *** = p = 0.0101.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1057 kb)
Supplementary Table 1
Antibodies. (XLS 33 kb)
Rights and permissions
About this article
Cite this article
Ulges, A., Klein, M., Reuter, S. et al. Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo. Nat Immunol 16, 267–275 (2015). https://doi.org/10.1038/ni.3083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3083
This article is cited by
-
Downregulation of LILRB4 Promotes Human Aortic Smooth Muscle Cell Contractile Phenotypic Switch and Apoptosis in Aortic Dissection
Cardiovascular Toxicology (2024)
-
Regulation of sclerostin by the SIRT1 stabilization pathway in osteocytes
Cell Death & Differentiation (2022)
-
CSNK2 in cancer: pathophysiology and translational applications
British Journal of Cancer (2022)
-
Elucidating different pattern of immunoregulation in BALB/c and C57BL/6 mice and their F1 progeny
Scientific Reports (2021)
-
Mechanisms of Allergen Immunotherapy in Allergic Rhinitis
Current Allergy and Asthma Reports (2021)