Abstract
Regulatory T cells (Treg cells) can express the transcription factors T-bet and GATA-3, but the function of this expression and whether such cells represent stable subsets is still unknown. By using various reporter tools, we found that the expression of T-bet and GATA-3 in Treg cells was dynamically influenced by the cytokine environment. Treg cell–specific deletion of the gene encoding either T-bet (Tbx21) or GATA-3 (Gata3) alone did not result in loss of Treg cell function; however, mice with combined deficiency in both genes in Treg cells developed severe autoimmune-like diseases. Loss of Treg cell function correlated with upregulation of expression of the transcription factor RORγt and reduced expression of the transcription factor Foxp3. Thus, in the steady state, activated Treg cells transiently upregulated either T-bet or GATA-3 to maintain T cell homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Lazarevic, V., Glimcher, L.H. & Lord, G.M. T-bet: a bridge between innate and adaptive immunity. Nat. Rev. Immunol. 13, 777–789 (2013).
Yagi, R., Zhu, J. & Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 23, 415–420 (2011).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Hwang, E.S, Szabo, S.J., Schwartzberg, P.L. & Glimcher, L.H. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307, 430–433 (2005).
Zhu, J. et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673 (2012).
Szabo, S.J., et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science 295, 338–342 (2002).
Zhang, F., Meng, G. & Strober, W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 9, 1297–1306 (2008).
Lazarevic, V. et al. T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 12, 96–104 (2011).
Josefowicz, S.Z., Lu, L.F. & Rudensky, A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Abbas, A.K. et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 14, 307–308 (2013).
Shevach, E.M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009).
Sakaguchi, S., Miyara, M., Costantino, C.M. & Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Chen, W. et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Zhou, L. et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Koch, M.A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Wohlfert, E.A. et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Invest. 121, 4503–4515 (2011).
Rudra, D. et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat. Immunol. 13, 1010–1019 (2012).
Wang, Y., Su, M.A. & Wan, Y.Y. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35, 337–348 (2011).
Wan, Y.Y. & Flavell, R.A. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA 102, 5126–5131 (2005).
Hosoya, T. et al. GATA-3 is required for early T lineage progenitor development. J. Exp. Med. 206, 2987–3000 (2009).
Duarte, J.H., Zelenay, S., Bergman, M.L., Martins, A.C. & Demengeot, J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 39, 948–955 (2009).
Koch, M.A. et al. T-bet+ Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity 37, 501–510 (2012).
Hall, A.O. et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37, 511–523 (2012).
Intlekofer, A.M. et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008).
Yagi, R. et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-γ. Immunity 32, 507–517 (2010).
Rubtsov, Y.P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008).
Izcue, A., Coombes, J.L. & Powrie, F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212, 256–271 (2006).
Delgoffe, G.M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Liston, A. & Gray, D.H. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 14, 154–165 (2014).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458, 351–356 (2009).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Chaudhry, A. et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 (2009).
Yamaguchi, T., Wing, J.B. & Sakaguchi, S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 23, 424–430 (2011).
Oldenhove, G. et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31, 772–786 (2009).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009).
Bailey-Bucktrout, S.L. et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013).
Thornton, A.M. & Shevach, E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998).
Levine, A.G., Arvey, A., Jin, W. & Rudensky, A.Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014).
Durbin, J.E., Hackenmiller, R., Simon, M.C. & Levy, D.E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84, 443–450 (1996).
Szymczak, A.L. et al. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589–594 (2004).
Indra, A.K. et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ERT and Cre-ERT2 recombinases. Nucleic Acids Res. 27, 4324–4327 (1999).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Sun, C.M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Acknowledgements
We thank J.D. Engel (University of Michigan) for GATA-3–GFP reporter mice on the C57BL/6 background; S. Reiner (University of Columbia) for Tbx21fl/fl mice; A. Rudensky (Memorial Sloan Kettering Cancer Center) for Foxp3-IRES-YFP-Cre mice; D. Jankovic (National Institute of Allergy and Infectious Diseases (NIAID)) for Stat1−/− mice; E. Shevach, Y. Belkaid and W. Paul for critical reading of the manuscript and discussions; A. Thornton for discussions; L. Guo for the help with enzyme-linked immunosorbent assay experiments; N. Cereb and S.Y. Yang (Histogenetics) and S. Reiner (Columbia University) for constructing and providing Tbx21fl/fl mice; and the flow cytometry core facility of the NIAID for cell sorting. Supported by the Division of Intramural Research of the NIAID (US National Institutes of Health).
Author information
Authors and Affiliations
Contributions
F.Y. performed all experiments; S.S. and J.E. helped in some experiments and made suggestions on the manuscript; L.F. helped make the transgenic mouse strains; and F.Y. and J.Z. designed the experiments, analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 AmCyan is a faithful reporter for T-bet expression.
(a) Splenocytes from the T-bet-AmCyan BAC transgenic mice were sorted for AmCyan+ and AmCyan- populations and then intracellular staining for T-bet expression in these two populations was carried out. (b) Naïve CD4+ (CD4+CD25-CD45RBhiAmCyan-) T cells were isolated by cell sorting from the T-bet-AmCyan BAC transgenic mice and then cultured under TH1 or TH2 polarizing conditions for 4 days. AmCyan was then assessed by flow cytometry. Each number above the gate indicates the percentage of AmCyan positive cells. Data are representative of two independent experiments.
Supplementary Figure 2 Analysis of Treg cell subsets before and after cell sorting.
(a) Splenocytes from the T-bet-AmCyan:GATA3-GFP:Foxp3-mRFP tri-color reporter mice were stained with anti-CD4 and anti-CXCR3. Plot on the left was gated on CD4+Foxp3-RFP+ population; histograms on the right show CXCR3 expression on each Treg subset. (b, c) Treg subsets were separated from the T-bet-AmCyan:GATA3-GFP:Foxp3-RFP tri-color reporter mice by cell sorting. The purity of each sorted population was shown (b). Plots were gated on CD4+Foxp3-RFP+ population. Some sorted cells were first stained with anti-CXCR3 and then fixed. They were then permeabilized and stained intracellularly with anti-T-bet overnight (c). Plots show T-bet and CXCR3 expression by each Treg subset. Data are representative of three (a), more than five (b) and two (c) independent experiments.
Supplementary Figure 3 T-bet expression in Treg cells depends on STAT1-activating cytokines.
Cells from the spleens and lymph nodes of wild type, Stat1-/-, Stat4-/- and IFN-gr1-/- T-bet-ZsGreen mice were fixed and stained for CD4, CD25 and Foxp3 and then analyzed by flow cytometry. Dot plots were gated on CD4+CD25+Foxp3+ population. Data are representative of two independent experiments.
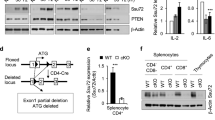
Supplementary Figure 4 Combined deficiency of T-bet and GATA-3 in Treg cells does not affect the development of Treg cells.
(a) Flow cytometric analysis of percentage of CD4+ and CD8+ T cells in the thymi of 8-week old Foxp3-Cre and Tbx21fl/flGata3fl/fl-Foxp3-Cre (DKO) mice. (b) Graphical presentation of percentage of Foxp3+ Treg cells among CD4 single positive thymocytes of 8-week old Foxp3-Cre and DKO mice. Each dot represents an individual mouse. (c) Graphical presentation of percentage of Foxp3+ Treg cells among CD4+ T cells in the spleen and lymph nodes of 8-week old Foxp3-Cre and DKO mice. Data are representative of two independent experiments. Error bars represent standard deviation of the mean (n=6). Statistical significance was determined by an ordinary one-way ANOVA (b and c). ns, not significant, *p<0.05, ****p<0.0001.
Supplementary Figure 5 Combined deficiency in T-bet and GATA-3 in Treg cells does not affect the proliferation or survival of Treg cells.
The splenocytes from 8-week old Foxp3-Cre and Tbx21fl/flGata3fl/fl-Foxp3-Cre (DKO) mice were stained with anti-CD4, anti-Foxp3, anti-CD25 and Ki-67 (a) or Bcl-2 (b). Dot plots were gated on CD4+Foxp3+ live cells. Numbers indicate the percentages in each quadrant. Data are representative of two independent experiments, each experiment with 2-3 mice in each group.
Supplementary Figure 6 Tbx21fl/flGata3fl/flFoxp3-Cre (DKO) Treg cells have normal expression of CD25 and CTLA-4 but lower expression of GITR, CD103 and Nrp-1.
The splenocytes from 8-week old Foxp3-Cre and DKO mice were stained with anti-CD4, anti-Foxp3 and antibodies against various “Treg-specific” cell surface markers. All the stainings were performed without cell permeablization except for CTLA-4 staining. Histograms were gated on CD4+Foxp3+ live cells. Data are representative of two independent experiments, each experiment with 2-3 mice in each group.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 426 kb)
Rights and permissions
About this article
Cite this article
Yu, F., Sharma, S., Edwards, J. et al. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol 16, 197–206 (2015). https://doi.org/10.1038/ni.3053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3053
This article is cited by
-
The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases
Nature Reviews Immunology (2024)
-
GATA-3 is a proto-oncogene in T-cell lymphoproliferative neoplasms
Blood Cancer Journal (2022)
-
Neutralization of interleukin-38 exacerbates coxsackievirus B3-induced acute myocarditis in mice
Virology Journal (2021)
-
Transcriptome and chromatin landscape of iNKT cells are shaped by subset differentiation and antigen exposure
Nature Communications (2021)
-
Wnt–β-catenin activation epigenetically reprograms Treg cells in inflammatory bowel disease and dysplastic progression
Nature Immunology (2021)