Abstract
Activated CD8+ T cells choose between terminal effector cell (TEC) or memory precursor cell (MPC) fates. We found that the signaling receptor Notch controls this 'choice'. Notch promoted the differentiation of immediately protective TECs and was correspondingly required for the clearance of acute infection with influenza virus. Notch activated a major portion of the TEC-specific gene-expression program and suppressed the MPC-specific program. Expression of Notch was induced on naive CD8+ T cells by inflammatory mediators and interleukin 2 (IL-2) via pathways dependent on the metabolic checkpoint kinase mTOR and the transcription factor T-bet. These pathways were subsequently amplified downstream of Notch, creating a positive feedback loop. Notch thus functions as a central hub where information from different sources converges to match effector T cell differentiation to the demands of an infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kaech, S.M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Joshi, N.S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Rutishauser, R.L. et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Jung, Y.W., Rutishauser, R.L., Joshi, N.S., Haberman, A.M. & Kaech, S.M. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J. Immunol. 185, 5315–5325 (2010).
Kallies, A., Xin, A., Belz, G.T. & Nutt, S.L. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009).
Cui, W., Liu, Y., Weinstein, J.S., Craft, J. & Kaech, S.M. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Rao, R.R., Li, Q., Gubbels Bupp, M.R. & Shrikant, P.A. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36, 374–387 (2012).
Ji, Y. et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat. Immunol. 12, 1230–1237 (2011).
Yang, C.Y. et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12, 1221–1229 (2011).
Intlekofer, A.M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Zhou, X. et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240 (2010).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813 (2009).
Rohr, J.C., Gerlach, C., Kok, L. & Schumacher, T.N. Single cell behavior in T cell differentiation. Trends Immunol. 35, 170–177 (2014).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Obar, J.J. et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 187, 4967–4978 (2011).
Chang, J.T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007).
Badovinac, V.P., Porter, B.B. & Harty, J.T. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5, 809–817 (2004).
Obar, J.J. et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA 107, 193–198 (2010).
Wiesel, M. et al. Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur. J. Immunol. 42, 320–329 (2012).
Bray, S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Kopan, R. & Ilagan, M.X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
Cho, O.H. et al. Notch regulates cytolytic effector function in CD8+ T cells. J. Immunol. 182, 3380–3389 (2009).
Kuijk, L.M. et al. Notch controls generation and function of human effector CD8+ T cells. Blood 121, 2638–2646 (2013).
Minter, L.M. et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 6, 680–688 (2005).
Bailis, W. et al. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity 39, 148–159 (2013).
Maekawa, Y. et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat. Immunol. 9, 1140–1147 (2008).
Belz, G.T., Xie, W., Altman, J.D. & Doherty, P.C. A previously unrecognized H-2Db-restricted peptide prominent in the primary influenza A virus-specific CD8+ T-cell response is much less apparent following secondary challenge. J. Virol. 74, 3486–3493 (2000).
Akira, S. & Hemmi, H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85, 85–95 (2003).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Rao, R.R., Li, Q., Odunsi, K. & Shrikant, P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78 (2010).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Helft, J. et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J. Clin. Invest. 122, 4037–4047 (2012).
Lambrecht, B.N. & Hammad, H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu. Rev. Immunol. 30, 243–270 (2012).
Braciale, T.J., Sun, J. & Kim, T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 12, 295–305 (2012).
Amsen, D. et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27, 89–99 (2007).
Olson, J.A., McDonald-Hyman, C., Jameson, S.C. & Hamilton, S.E. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity 38, 1250–1260 (2013).
Adler, S.H. et al. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J. Immunol. 171, 2896–2903 (2003).
Kim, E.H. et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J. Immunol. 188, 4305–4314 (2012).
Macintyre, A.N. et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 34, 224–236 (2011).
Ciofani, M. & Zuniga-Pflucker, J.C. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 6, 881–888 (2005).
Palomero, T. et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 13, 1203–1210 (2007).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Ito, T. et al. The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection. PLoS Pathog. 7, e1002341 (2011).
Chastagner, P., Israel, A. & Brou, C. AIP4/Itch regulates Notch receptor degradation in the absence of ligand. PLoS ONE 3, e2735 (2008).
Koo, B.K. et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development 132, 3459–3470 (2005).
Guruharsha, K.G., Kankel, M.W. & Artavanis-Tsakonas, S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666 (2012).
Demarest, R.M., Ratti, F. & Capobianco, A.J. It's T-ALL about Notch. Oncogene 27, 5082–5091 (2008).
Cantrell, D. Protein kinase B (Akt) regulation and function in T lymphocytes. Semin. Immunol. 14, 19–26 (2002).
Hand, T.W. et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl. Acad. Sci. USA 107, 16601–16606 (2010).
Pipkin, M.E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010).
Finlay, D.K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Doedens, A.L. et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 (2013).
Topham, D.J., Castrucci, M.R., Wingo, F.S., Belz, G.T. & Doherty, P.C. The role of antigen in the localization of naive, acutely activated, and memory CD8+ T cells to the lung during influenza pneumonia. J. Immunol. 167, 6983–6990 (2001).
Mueller, S.N., Langley, W.A., Carnero, E., Garcia-Sastre, A. & Ahmed, R. Immunization with live attenuated influenza viruses that express altered NS1 proteins results in potent and protective memory CD8+ T-cell responses. J. Virol. 84, 1847–1855 (2010).
van der Sluijs, K.F. et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 172, 7603–7609 (2004).
Palmer, D.F., Dowdle, W.R., Coleman, M.T. & Schild, G.C. in Atlanta: US Department of Health, Education and Welfare, Public Health Service 25–62 (US Department of Health, Education and Welfare, Public Health Service, 1975).
Anders, S. et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc. 8, 1765–1786 (2013).
Young, M.D., Wakefield, M.J., Smyth, G.K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 (2010).
Isserlin, R. et al. Pathway analysis of dilated cardiomyopathy using global proteomic profiling and enrichment maps. Proteomics 10, 1316–1327 (2010).
Acknowledgements
We thank J.C. Zuniga Pflucker (Sunnybrook Research Institute) and D. Vignali (University of Pittsburgh) for the pMIY and pMIY-myr-Akt retroviral expression constructs; T.N.M. Schumacher (Netherlands Cancer Institute) for OVA-influenza; J. den Haan (Free University of Amsterdam) for MyD88-deficient mice; M. Suresh and E.H. Kim for advice on the staining of phosphorylated proteins for flow cytometry; M. Wolkers, M. Nolte, R. van Lier and M. van Ham for suggestions and proofreading of the manuscript; and the tetramer facility of the US National Institutes of Health for tetramers of major histocompatibility complex. Supported by NWO-ALW, the Landsteiner Foundation for Blood Research, the Academic Medical Center (D.A.), the US National Institutes of Health (AI095245 and DK072201 to J.M.B.), the Burroughs Wellcome Trust Fund (J.M.B.), the Leukemia and Lymphoma Society (J.M.B.), the Irma-Hirschl Charitable Trust and Monique Weill-Caulier Charitable Trust (J.M.B.).
Author information
Authors and Affiliations
Contributions
R.A.B. designed, performed and analyzed experiments and wrote the manuscript; C.H., R.G., A.K., B.J.L., C.X.D., Y.S.d.S., S.E.v.T., R.v.B. and A.t.B. performed and analyzed experiments; A.M.W. and A.H.C.v.K. analyzed data from high-throughput RNA sequencing; G.F.R., S.M.K., J.M.B. and K.v.G. designed and analyzed experiments; and D.A. supervised the study, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 TLR ligands induce a soluble factor that promotes the expression of Notch in CD8+ T cells.
(a) Naïve CD8+ T cells were stimulated with 10 µg/ml plate bound anti-CD3 alone (black bars) or in the presence of R-848 (white bars) or LPS (grey bars) without DC. Cell surface expression of Notch1 (left) and Notch2 (right) was determined after 16 h of culture by FACS and displayed as geo Mean Fluorescence Intensities (MFI) + s.e.m. (b) Naïve CD8+ T cells were stimulated o/n with plate-bound anti-CD3 mAb with supernatants from unstimulated (black bars), R-848 stimulated (white bars) or LPS stimulated (grey bars) WT BM-DC (left) or MyD88ko BM-DC cultures (right) and average Notch1 surface expression was determined by FACS. (c) Naïve CD8+ T cells, isolated from WT (black bars), MyD88ko (white bars) or Notch1-2-KO mice (grey bars), were stimulated o/n with plate bound anti-CD3 mAb and supernatants isolated from non-stimulated (DC sup) or R-848 stimulated BM-DC cultures (R-848 DC sup). Expression of Notch1 was determined as in (a). Triplicates, representative of 2 experiments. *** P < 0.001, One-way ANOVA with Bonferroni corrections.
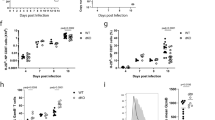
Supplementary Figure 2 Notch surface levels are elevated on early TECs in vivo.
Mice were infected with HKx31 influenza, and after 3 days, different CD8+ T cell subpopulations (grey bars-CD44+KLRG1+; white bars-CD44+KLRG1–; black bars-CD44–) were stained for N1 and N2 expression. Results represent 4 pooled mice. Shown are mean + s.e.m. * P < 0.05; ** P < 0.01; unpaired, two-tailed t-test.
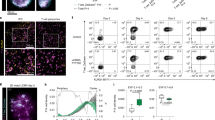
Supplementary Figure 3 Expression of Notch ligands on mediastinal lymph nodes and lung APCs during infection with influenza virus.
C57BL/6 mice were infected intranasally with A/HKx31 influenza. (a) Five days post infection (p.i.) expression of DLL1, DLL4, Jagged1 and Jagged2 expression on mediastinal LN (mLN) APC subsets was measured by flow cytometry. Two main APC subsets in the mLN were defined as mDCs (migratory DC, MHCIIhiCD11c+, red gate) or macrophages (MΦ, MHCIImedCD11chi, green gate). (b) Bar graph shows average cell surface expression of DLL1, DLL4, Jagged1 and Jagged2 on mLN macrophages. Shown is Δ-MFI (corrected for background staining with isotype control mAb) for uninfected (UI-white bars) and infected mice (black bars). (c) Gating strategy for SSChiMHCIImedCD11c- cells (gate I), alveolar MΦ (SSChiMHCIImedCD11chi, gate I), and mDCs (migratory DC, MHCIIhiCD11chi, gate III) in lung following (Helft, J. et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest 122, 4037-4047 (2012).), 5 days post infection (p.i.). Expression of DLL1, DLL4, Jagged1 and Jagged2 on these cell subsets was measured by flow cytometry. Bar graphs represent average Δ-MFI (corrected for background staining with isotype control mAb) for non-infected (white bars) and infected mice (black bars). Results represent 2 (uninfected) or 4 (infected) separately processed mice from a representative of 5 experiments. Shown are the mean + s.e.m. * P < 0.05; two-tailed t-test.
Supplementary Figure 4 TEC differentiation is dependent on Notch gene dose.
Notch1flox/+Notchflox/+ wild type (grey bars) or Notch1flox/+Notchflox/+ CD4-Cre+ double heterozygous littermate mice (open bars) were infected with A/HkX31 influenza and after 10 days the expression of KLRG1 and CD127 was determined on H-2Db–NP366-374 binding CD8+ T cells. Results are cumulative from 2 experiments with 10 mice per group total. Shown are the mean + s.e.m. (* P < 0.05; two-tailed t-test).
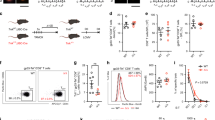
Supplementary Figure 5 Efficient CD8+ T cell effector functions during viral infection depend on RBPJ.
RBPJflox/floxCd4-Cre+ (RBPJ-KO) and WT control littermate mice were infected intranasally with A/HKx31 influenza. Ten days post-infection, CD8+ T cell responses were analyzed. (a) Average frequencies of H-2Db–NP+ CD8+ T cells in blood, 10 days after infection. Black bars represent WT and white bars RBPJ-KO mice. (b) Single cell suspensions from lungs of WT and RBPJ-KO mice, were stimulated with NP366-374 peptide for 4 hours in the presence of brefeldin A. Percentages of CD8+ T cells producing IFNγ (left) and TNF (right) are shown. (3 mice/group, representative experiment of 2). * P < 0.05; two-tailed t-test.
Supplementary Figure 6 The role of Notch in TEC differentiation is independent of the strain of influenza virus.
Mice were infected intranasally with A/PR/8/34 (200xTCID50) and analyzed after 10 days. (a) Representative FACS profiles and (b) average frequencies of H-2 Db–NP-binding CD8+ T cells in blood from WT (grey bars) and Notch1-2-KO (white bars) mice. (c) Average frequencies (left) and total cell numbers (right) of H-2 Db–NP+CD8+ T cells in spleens and lungs. (d) Representative FACS profiles for KLRG1 and CD127 and (e) percentages of H-2 Db–NP+CD8+ TECs and MPCs in blood, spleens and lungs from wild type (grey bars) and Notch1-2-KO (white bars) mice. (f) FACS profile for Granzyme-B production by WT (black line) and Notch1-2-KO (grey line) lung CD8+ T cells after NP366-374 peptide stimulation. Filled grey histogram represents isotype control staining. (g) Average percentages of CD8+ T cells from lungs producing (from left to right) GZMB, IFNγ and TNF after stimulation with NP366-374 peptide. Shown are mean + s.e.m. of 4 mice per group. * P < 0.05; ** P < 0.01; *** P<0.001; unpaired, two-tailed t-test.
Supplementary Figure 7 The requirement for Notch in TEC differentiation is independent of TCR specificity.
Seven hundred fifty wild type (grey bars) or Notch1-2-KO (white bars) OT-I CD45.2+ CD8+ T cells were adoptively transferred into CD45.1 congenic wild type mice infected with WSN-Ova. After 10 days, the percentage of CD45+ OT-I cells among CD8+ T cells (left) or KLRG1+ cells among the OT-I T cells (right) was determined by FACS. Result is representative of 3 experiments. *** P<0.001.
Supplementary Figure 8 Notch controls pathways that promote TEC differentiation.
(a) Phosphorylation of FoxO1/3 is reduced in Notch1-2-KO CD8 T cells. FACS profile (left) and average Δ-MFI (right - corrected for background staining) for Phosphorylation of FoxO1/3 (Thr24/Thr32) in transferred WT (black line, grey bars) and Notch1-2-KO (grey line, white bars) OT-I T cells, 5 days post-infection with OVA-influenza. Black bars indicate pFoxO1/3 staining on CD44–CD8+ T cells. Results are cumulative from two different experiments with a total of 8 individually tested mice per group. * P < 0.05, unpaired, two-tailed t-test. (b) Model showing signal integration and positive feedback of the Notch hub.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 635 kb)
Supplementary Dataset 1
Gene sets GSEA (XLSX 298 kb)
Supplementary Dataset 2
TEC and MPC genes (XLSX 143 kb)
Supplementary Dataset 3
Go categories (XLS 17 kb)
Rights and permissions
About this article
Cite this article
Backer, R., Helbig, C., Gentek, R. et al. A central role for Notch in effector CD8+ T cell differentiation. Nat Immunol 15, 1143–1151 (2014). https://doi.org/10.1038/ni.3027
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3027
This article is cited by
-
FOXP1 and KLF2 reciprocally regulate checkpoints of stem-like to effector transition in CAR T cells
Nature Immunology (2024)
-
Reprogramming T cell differentiation and exhaustion in CAR-T cell therapy
Journal of Hematology & Oncology (2023)
-
A data-driven Boolean model explains memory subsets and evolution in CD8+ T cell exhaustion
npj Systems Biology and Applications (2023)
-
Notch signaling pathway: architecture, disease, and therapeutics
Signal Transduction and Targeted Therapy (2022)
-
High-fat diet increases the severity of Giardia infection in association with low-grade inflammation and gut microbiota dysbiosis
Scientific Reports (2021)