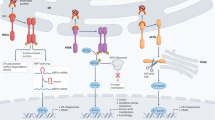
Abstract
The unfolded protein response (UPR) has traditionally been viewed as an adaptive response triggered by the accumulation of unfolded proteins in the endoplasmic reticulum (ER) and aimed at restoring ER function. The UPR can also be an anticipatory response that is activated well before the disruption of protein homeostasis. UPR signaling intersects at many levels with the innate and adaptive immune responses. In some types of cells of the immune system, such as dendritic cells (DCs) and B cells, particular sensors that detect the UPR seem to be constitutively active in the absence of induction of the traditional UPR gene program and are necessary for antigen presentation and immunoglobulin synthesis. The UPR also influences signaling via Toll-like receptors (TLRs) and activation of the transcription factor NF-κB, and some pathogens subvert the UPR. This Review summarizes these emerging noncanonical functions of the UPR in immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Kim Caesar/Nature Publishing Group

Kim Caesar/Nature Publishing Group

Kim Caesar/Nature Publishing Group

Kim Caesar/Nature Publishing Group
Similar content being viewed by others
References
Araki, K. & Nagata, K. Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 4, a015438 (2012).
Smith, M.H., Ploegh, H.L. & Weissman, J.S. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, 1086–1090 (2011).
Ma, Y. & Hendershot, L.M. The stressful road to antibody secretion. Nat. Immunol. 4, 310–311 (2003).
Harding, H.P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999). First paper of a series of studies delineating the role of PERK in regulating protein translation in response to ER stress.
Hollien, J. et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331 (2009).
Hollien, J. & Weissman, J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006). First study to demonstrate the role of IRE-1 in a previously unanticipated function of controlling mRNA degradation.
Travers, K.J. et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 (2000).
Lin, J.H. et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 (2007).
Cox, J.S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001). Nearly simultaneous discovery by the Walter group (ref. 9 ) and the Mori group (ref. 10 and other studies by the Mori group) of the role of XBP-1 in the UPR.
Shaffer, A.L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004).
Shoulders, M.D. et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep 3, 1279–1292 (2013).
Sriburi, R., Jackowski, S., Mori, K. & Brewer, J.W. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35–41 (2004).
Yoshida, H. et al. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell 4, 265–271 (2003).
Kimmig, P. et al. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. eLife 1, e00048 (2012). Study demonstrating that the ancestral function of IRE-1 was probably RIDD and not a transcriptional response.
Korennykh, A. & Walter, P. Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 28, 251–277 (2012).
Ghosh, R. et al. Allosteric inhibition of the IRE1a RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548 (2014).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Bertolotti, A. et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1b-deficient mice. J. Clin. Invest. 107, 585–593 (2001).
Martino, M.B. et al. The ER stress transducer IRE1b is required for airway epithelial mucin production. Mucosal Immunol. 6, 639–654 (2013).
Tsuru, A. et al. Negative feedback by IRE1b optimizes mucin production in goblet cells. Proc. Natl. Acad. Sci. USA 110, 2864–2869 (2013).
Iqbal, J. et al. IRE1b inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 7, 445–455 (2008).
Iwawaki, T. et al. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat. Cell Biol. 3, 158–164 (2001).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999). First in a series of studies that together identified the role of ATF6 in the UPR.
Yoshida, H., Haze, K., Yanagi, H., Yura, T. & Mori, K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273, 33741–33749 (1998).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Adachi, Y. et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 33, 75–89 (2008).
Bommiasamy, H. et al. ATF6a induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 122, 1626–1636 (2009).
Kondo, S., Saito, A., Asada, R., Kanemoto, S. & Imaizumi, K. Physiological unfolded protein response regulated by OASIS family members, transmembrane bZIP transcription factors. IUBMB Life 63, 233–239 (2011).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006).
Shi, Y. et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 a-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 (1998).
Harding, H.P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Hinnebusch AG, Lorsch JR. The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harbor Perspect. Bio. 4, a011544 (2012).
Harding, H.P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Novoa, I. et al. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 22, 1180–1187 (2003).
Harding, H.P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Hinnebusch, A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59, 407–450 (2005).
Bertolotti, A., Zhang, Y., Hendershot, L.M., Harding, H.P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 (2000).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Pincus, D. et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 (2010).
Kimata, Y., Oikawa, D., Shimizu, Y., Ishiwata-Kimata, Y. & Kohno, K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 167, 445–456 (2004).
Credle, J.J., Finer-Moore, J.S., Papa, F.R., Stroud, R.M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 102, 18773–18784 (2005).
Gardner, B.M. & Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 (2011). Seminal paper presenting an alternative model for IRE-1 activation through direct binding of unfolded proteins.
Liu, C.Y., Schroder, M. & Kaufman, R.J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 275, 24881–24885 (2000).
Nadanaka, S., Okada, T., Yoshida, H. & Mori, K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol. Cell. Biol. 27, 1027–1043 (2007).
Rutkowski, D.T. & Hegde, R.S. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 189, 783–794 (2010).
Iwakoshi, N.N. et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4, 321–329 (2003). One of the earliest papers showing an essential role for XBP-1 in cells of the immune system.
van Anken, E. et al. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18, 243–253 (2003).
Hu, C.C., Dougan, S.K., McGehee, A.M., Love, J.C. & Ploegh, H.L. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 28, 1624–1636 (2009).
Osorio, F. et al. The unfolded-protein-response sensor IRE-1α regulates the function of CD8alpha dendritic cells. Nat. Immunol. 15, 248–257 (2014). First paper demonstrating a role for the IRE-1–XBP-1 branch in the presentation of antigen by MHC class I in DCs via the RIDD pathway.
Brunsing, R. et al. B- and T-cell development both involve activity of the unfolded protein response pathway. J. Biol. Chem. 283, 17954–17961 (2008).
Martinon, F., Chen, X., Lee, A.-H. & Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 (2010).
Woo, C.W., Kutzler, L., Kimball, S.R. & Tabas, I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat. Cell Biol. 14, 192–200 (2012).
Volmer, R., van der Ploeg, K. & Ron, D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. USA 110, 4628–4633 (2013).
Wiseman, R.L. et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol. Cell 38, 291–304 (2010). First study demonstrating activation of IRE-1 not via its luminal domain but via its cytoplasmic domain, suggesting the existence of alternative ways to trigger sensors that detect the UPR.
Hollien, J. Evolution of the unfolded protein response. Biochim. Biophys. Acta 1833, 2458–2463 (2013).
Askew, D.S. Endoplasmic reticulum stress and fungal pathogenesis converge. Virulence 5, 331–333 (2014).
Nagashima, Y. et al. Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci. Rep. 1, 29 (2011).
Liu, J.X., Srivastava, R., Che, P. & Howell, S.H. An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 19, 4111–4119 (2007).
Martínez, I.M. & Chrispeels, M.J. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15, 561–576 (2003).
Moreno, A.A. et al. IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS ONE 7, e31944 (2012).
Tateda, C. et al. NtbZIP60, an endoplasmic reticulum-localized transcription factor, plays a role in the defense response against bacterial pathogens in Nicotiana tabacum. J. Plant Res. 121, 603–611 (2008).
Jelitto-Van Dooren, E.P., Vidal, S. & Denecke, J. Anticipating endoplasmic reticulum stress. A novel early response before pathogenesis-related gene induction. Plant Cell 11, 1935–1944 (1999).
Shen, X. et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107, 893–903 (2001).
Richardson, C.E., Kooistra, T. & Kim, D.H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463, 1092–1095 (2010). Elegant study demonstrating that in C. elegans , XBP-1 tempers an otherwise devastating immune response.
Adolph, T.E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013). One of several papers elucidating the role of the IRE-1–XBP-1 branch in mucosal immunology.
Tattoli, I. et al. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11, 563–575 (2012). Very elegant study demonstrating how the ISR and innate host defense responses are very closely linked.
Clavarino, G. et al. Induction of GADD34 is necessary for dsRNA-dependent interferon-beta production and participates in the control of Chikungunya virus infection. PLoS Pathog. 8, e1002708 (2012).
Ravindran, R. et al. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 343, 313–317 (2014). First study to delineate the role of GCN2 in enhancing autophagy and antigen presentation in DCs.
Lemaitre, B. & Girardin, S.E. Translation inhibition and metabolic stress pathways in the host response to bacterial pathogens. Nat. Rev. Microbiol. 11, 365–369 (2013).
Harding, H.P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Zhang, P. et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 (2002).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Lee, A.H., Chu, G.C., Iwakoshi, N.N. & Glimcher, L.H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005).
Reimold, A.M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Todd, D.J. et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 206, 2151–2159 (2009).
Benhamron, S. et al. Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur. J. Immunol. 44, 867–876 (2014).
Iwakoshi, N.N., Pypaert, M. & Glimcher, L.H. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 204, 2267–2275 (2007).
Iwawaki, T., Akai, R., Kohno, K. & Miura, M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10, 98–102 (2004). Development of a reporter gene tool that appeared extremely useful for monitoring IRE-1 activation in physiological conditions in vivo.
Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Osorio, F., Lambrecht, B. & Janssens, S. The UPR and lung disease. Semin. Immunopathol. 35, 293–306 (2013).
Adolph, T.E., Niederreiter, L., Blumberg, R.S. & Kaser, A. Endoplasmic reticulum stress and inflammation. Dig. Dis. 30, 341–346 (2012).
Engin, F. et al. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci. Transl. Med. 2013, 5, 211ra156 (2013).
Zhang, K. & Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462 (2008).
Hasnain, S.Z., Lourie, R., Das, I., Chen, A.C.-H. & McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 90, 260–270 (2012).
Jiang, H.Y. et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NFκB in response to diverse cellular stresses. Mol. Cell. Biol. 23, 5651–5663 (2003).
Hu, P., Han, Z., Couvillon, A.D., Kaufman, R.J. & Exton, J.H. Autocrine tumor necrosis factor a links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1a-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084 (2006).
Kaneko, M., Niinuma, Y. & Nomura, Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 26, 931–935 (2003).
Yamazaki, H. et al. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 183, 1480–1487 (2009).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Nakamura, T. et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140, 338–348 (2010).
Petrasek, J. et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. USA 110, 16544–16549 (2013).
Gora, S. et al. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 24, 3284–3297 (2010).
Maguire, J.A., Mulugeta, S. & Beers, M.F. Endoplasmic reticulum stress induced by surfactant protein C BRICHOS mutants promotes proinflammatory signaling by epithelial cells. Am. J. Respir. Cell Mol. Biol. 44, 404–414 (2011).
Marjon, P.L., Bobrovnikova-Marjon, E.V. & Abcouwer, S.F. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol. Cancer 3, 4 (2004).
Lerner, A.G. et al. IRE1a induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264 (2012).
Hu, F. et al. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. Eur. J. Immunol. 41, 1086–1097 (2011).
Goodall, J.C. et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA 107, 17698–17703 (2010).
Martino, M.E.B. et al. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J. Biol. Chem. 284, 14904–14913 (2009).
Smith, J.A. et al. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-b induction via X-box binding protein 1. Eur. J. Immunol. 38, 1194–1203 (2008).
Dalod, M. & Pierre, P. Integration of ER stress and viral nucleotide sensing in DCs: mounting a response commensurate to the threat? Eur. J. Immunol. 41, 898–901 (2011).
Hayakawa, K. et al. Acquisition of anergy to proinflammatory cytokines in nonimmune cells through endoplasmic reticulum stress response: a mechanism for subsidence of inflammation. J. Immunol. 182, 1182–1191 (2009).
Li, J., Wang, J.J. & Zhang, S.X. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J. Biol. Chem. 286, 4912–4921 (2011).
Kitamura, M. Control of NF-κB and inflammation by the unfolded protein response. Int. Rev. Immunol. 30, 4–15 (2011).
Hayakawa, K. et al. ER stress depresses NF-κB activation in mesangial cells through preferential induction of C/EBPb. J. Am. Soc. Nephrol. 21, 73–81 (2010).
Arensdorf, A.M. & Rutkowski, D.T. Endoplasmic reticulum stress impairs IL-4/IL-13 signaling through C/EBPb-mediated transcriptional suppression. J. Cell Sci. 126, 4026–4036 (2013).
Liang, Q., Deng, H., Sun, C.W., Townes, T.M. & Zhu, F. Negative regulation of IRF7 activation by activating transcription factor 4 suggests a cross-regulation between the IFN responses and the cellular integrated stress responses. J. Immunol. 186, 1001–1010 (2011).
He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13, 393–403 (2006).
Roy, C.R., Salcedo, S.P. & Gorvel, J.P. Pathogen-endoplasmic-reticulum interactions: in through the out door. Nat. Rev. Immunol. 6, 136–147 (2006).
Cho, J.A. et al. The unfolded protein response element IRE1a senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe 13, 558–569 (2013).
Pillich, H., Loose, M., Zimmer, K.P. & Chakraborty, T. Activation of the unfolded protein response by Listeria monocytogenes. Cell. Microbiol. 14, 949–964 (2012).
Ambrose, R.L. & Mackenzie, J.M. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J. Virol. 85, 2723–2732 (2011).
Hassan, I. et al. Inositol-requiring enzyme 1 inhibits respiratory syncytial virus replication. J. Biol. Chem. 289, 7537–7546 (2014).
Hassan, I.H. et al. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 287, 4679–4689 (2012).
Tardif, K.D., Mori, K., Kaufman, R.J. & Siddiqui, A. Hepatitis C virus suppresses the IRE1–XBP1 pathway of the unfolded protein response. J. Biol. Chem. 279, 17158–17164 (2004).
Stahl, S. et al. Cytomegalovirus downregulates IRE1 to repress the unfolded protein response. PLoS Pathog. 9, e1003544 (2013).
Zheng, Y. et al. Hepatitis C virus non-structural protein NS4B can modulate an unfolded protein response. J. Microbiol. 43, 529–536 (2005).
Woo, C.W. et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat. Cell Biol. 11, 1473–1480 (2009). One of the first papers to demonstrate that TLR signaling modifies the UPR response.
Nakayama, Y. et al. Molecular mechanisms of the LPS-induced non-apoptotic ER stress-CHOP pathway. J. Biochem. 147, 471–483 (2010).
Cláudio, N., Dalet, A., Gatti, E. & Pierre, P. Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J. 32, 1214–1224 (2013).
Ke, P.Y. & Chen, S.S. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 121, 37–56 (2011).
Bhattacharyya, S., Sen, U. & Vrati, S. Regulated IRE1-dependent decay pathway is activated during Japanese encephalitis virus-induced unfolded protein response and benefits viral replication. J. Gen. Virol. 95, 71–79 (2014).
Liou, H.C. et al. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science 247, 1581–1584 (1990).
Qian, S.B. et al. Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. J. Immunol. 177, 227–233 (2006).
Granados, D.P. et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 10, 10 (2009).
Schwab, S.R., Shugart, J.A., Horng, T., Malarkannan, S. & Shastri, N. Unanticipated antigens: translation initiation at CUG with leucine. PLoS Biol. 2, e366 (2004).
Nakaya, H.I. et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 12, 786–795 (2011). Large systems-biology approach to identify molecular signatures upon vaccination that correlate with and could be used to predict later antibody responses.
Krysko, D.V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Narasimhan, J. et al. Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J. Biol. Chem. 283, 16591–16601 (2008).
Hur, K. et al. IRE1a activation protects mice against acetaminophen-induced hepatotoxicity. J. Exp. Med. 209, 307–318 (2012). First studies (refs. 131,132 ) to show hyperactivation of IRE-1α and hence RIDD activity in XBP-1 deficiency, explaining some of the phenotypes observed in XBP-1 deficiency.
Han, D. et al. IRE1a kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 (2009). Study showing that with artificially designed IRE-1 constructs it is possible to regulate RIDD and XBP-1 splicing activity independently.
Donnelly, N., Gorman, A.M., Gupta, S. & Samali, A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013).
Wek, R.C., Jiang, H.Y. & Anthony, T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34, 7–11 (2006).
Baird, T.D. & Wek, R.C. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 3, 307–321 (2012).
Anderson, P. & Kedersha, N. Stress granules. Curr. Biol. 19, R397–R398 (2009).
Maurel, M., Chevet, E., Tavernier, J. & Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Upton, J.P. et al. IRE1a cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 338, 818–822 (2012).
Lee, A.-H., Heidtman, K., Hotamisligil, G. & Glimcher, L. Dual and opposing roles of the unfolded protein response regulated by IRE1a and XBP1 in proinsulin processing and insulin secretion. Proc. Natl. Acad. Sci. USA 108, 8885–8890 (2011).
Eckard, S.C. et al. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 15, 839–845 (2014). Study showing that much as the exonuclease Trex1 metabolizes self DNA to avoid autoimmunity, the RNA exosome neutralizes RNA fragments generated by the RIDD pathway that trigger RIG-I receptors.
Acknowledgements
We thank F. Martinon, P. de Bleser, P. Hulpiau and L. Martens for comments on the bioinformatics analysis. Supported by the European Research Council (B.N.L.), Fonds Wetenschappelijk Onderzoek (B.N.L. and S.J.), University of Ghent Multidisciplinary Research Platform (B.N.L and S.J.), the US National Institutes of Health (R37 DK057665, R37 AI048638, U19 AI090023 and U19 AI057266 to B.P.) and the Bill & Melinda Gates Foundation (B.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Janssens, S., Pulendran, B. & Lambrecht, B. Emerging functions of the unfolded protein response in immunity. Nat Immunol 15, 910–919 (2014). https://doi.org/10.1038/ni.2991
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2991
This article is cited by
-
Emerging concepts in the science of vaccine adjuvants
Nature Reviews Drug Discovery (2021)
-
Reprogramming of microRNA expression via E2F1 downregulation promotes Salmonella infection both in infected and bystander cells
Nature Communications (2021)
-
Dicarbonyl stress, protein glycation and the unfolded protein response
Glycoconjugate Journal (2021)
-
Unfolded protein response during cardiovascular disorders: a tilt towards pro-survival and cellular homeostasis
Molecular and Cellular Biochemistry (2021)
-
Perturbation of ubiquitin homeostasis promotes macrophage oxidative defenses
Scientific Reports (2019)