Abstract
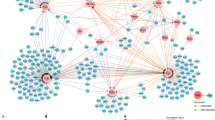
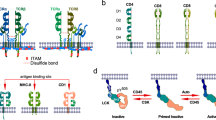
The activation of T cells mediated by the T cell antigen receptor (TCR) requires the interaction of dozens of proteins, and its malfunction has pathological consequences. Our major focus is on new developments in the systems-level understanding of the TCR signal-transduction network. To make sense of the formidable complexity of this network, we argue that 'fine-grained' methods are needed to assess the relationships among a few components that interact on a nanometric scale, and those should be integrated with high-throughput '-omic' approaches that simultaneously capture large numbers of parameters. We illustrate the utility of this integrative approach with the transmembrane signaling protein Lat, which is a key signaling hub of the TCR signal-transduction network, as a connecting thread.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
25 August 2014
In the version of this article initially published, the page ranges for references 7 and 15 were missing. Those are 815-823 (ref. 7) and 808-814 (ref. 15). The error has been corrected in the HTML and PDF versions of the article.
References
Huang, J. et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4+ T cells. Immunity 39, 846–857 (2013).Two key papers (refs. 1 and 3) showing that a single peptide-MHC suffices to trigger T cell activation.
Manz, B.N., Jackson, B.L., Petit, R.S., Dustin, M.L. & Groves, J. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc. Natl. Acad. Sci. USA 108, 9089–9094 (2011).
O'Donoghue, G.P., Pielak, R.M., Smoligovets, A.A., Lin, J.J. & Groves, J.T. Direct single molecule measurement of TCR triggering by agonist pMHC in living primary T cells. eLife 2, e00778 (2013).
Houtman, J.C., Houghtling, R.A., Barda-Saad, M., Toda, Y. & Samelson, L.E. Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J. Immunol. 175, 2449–2458 (2005).
Huse, M. et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity 27, 76–88 (2007).Two key papers (refs. 5 and 6) showing the high speed at which TCR signaling proceeds.
Brodovitch, A., Bongrand, P. & Pierres, A. T lymphocytes sense antigens within seconds and make a decision within one minute. J. Immunol. 191, 2064–2071 (2013).
Hogquist, K.A.J. & Jameson, S.C. The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function. Nat. Immunol. 15, 815–823 (2014).
Pitcher, L.A. et al. The formation and functions of the 21- and 23-kDa tyrosine-phosphorylated TCR zeta subunits. Immunol. Rev. 191, 47–61 (2003).
Moran, A.E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Hochweller, K. et al. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc. Natl. Acad. Sci. USA 107, 5931–5936 (2010).
Huppa, J.B. & Davis, M.M. The interdisciplinary science of T-cell recognition. Adv. Immunol. 119, 1–50 (2013).
Chakraborty, A.K.W. & Weiss, A. Insights into the initiation of TCR signaling. Nat. Immunol. 15, 815––823 (2014).
Sangani, D., Venien-Bryan, C. & Harder, T. Phosphotyrosine-dependent in vitro reconstitution of recombinant LAT-nucleated multiprotein signalling complexes on liposomes. Mol. Membr. Biol. 26, 159–170 (2009).
Roncagalli, R. et al. Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub. Nat. Immunol. 15, 384–392 (2014).Key paper describing the Zap70–Lat–SLP-76 interactome in primary CD4+ T cells.
Navarro, M.N.C. & Cantrell, D. Serine-threonine kinases in TCR signaling. Nat. Immunol. 15, 808–814 (2014).
Mingueneau, M. et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity 31, 197–208 (2009).
Salek, M. et al. Quantitative phosphoproteome analysis unveils LAT as a modulator of CD3zeta and ZAP-70 tyrosine phosphorylation. PLoS ONE 8, e77423 (2013).
Ou-Yang, C.W. et al. Role of LAT in the granule-mediated cytotoxicity of CD8 T cells. Mol. Cell. Biol. 32, 2674–2684 (2012).
Pagán, A.J., Pepper, M., Chu, H.H., Green, J.M. & Jenkins, M.K. CD28 promotes CD4+ T cell clonal expansion during infection independently of its YMNM and PYAP motifs. J. Immunol. 189, 2909–2917 (2012).
Kong, K.F. et al. A motif in the V3 domain of the kinase PKC-θ determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat. Immunol. 12, 1105–1112 (2011).
Chuang, H.C. et al. The kinase GLK controls autoimmunity and NF-κB signaling by activating the kinase PKC-θ in T cells. Nat. Immunol. 12, 1113–1118 (2011).
Liang, Y. et al. The lymphoid lineage-specific actin-uncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat. Immunol. 14, 858–866 (2013).
Michel, F. & Acuto, O. CD28 costimulation: a source of Vav-1 for TCR signaling with the help of SLP-76? Sci. STKE 2002, pe35 (2002).
Kim, J.E. & White, F.M. Quantitative analysis of phosphotyrosine signaling networks triggered by CD3 and CD28 costimulation in Jurkat cells. J. Immunol. 176, 2833–2843 (2006).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8, 89–95 (1998).
Chen, L. & Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013).
Simpson, T.R. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013).
van Panhuys, N., Klauschen, F. & Germain, R.N. T cell receptor-dependent signal intensity dominantly controls CD4+ T cell polarization in vivo. Immunity 41, 63–74 (2014).
Varjosalo, M. et al. Interlaboratory reproducibility of large-scale human protein-complex analysis by standardized AP-MS. Nat. Methods 10, 307–314 (2013).
Zheng, Y. et al. Temporal regulation of EGF signalling networks by the scaffold protein Shc1. Nature 499, 166–171 (2013).Key paper analyzing the causal relationships within a complex signal-transduction network.
Mayya, V. et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci. Signal. 2, ra46 (2009).
Mbonye, U.R. et al. Phosphorylation of CDK9 at Ser175 enhances HIV transcription and is a marker of activated P-TEFb in CD4+ T lymphocytes. PLoS Pathog. 9, e1003338 (2013).
Astoul, E., Edmunds, C., Cantrell, D.A. & Ward, S.G. PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol. 22, 490–496 (2001).
Brockmeyer, C. et al. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J. Biol. Chem. 286, 7535–7547 (2011).
Navarro, M.N., Goebel, J., Feijoo-Carnero, C., Morrice, N. & Cantrell, D.A. Phosphoproteomic analysis reveals an intrinsic pathway for the regulation of histone deacetylase 7 that controls the function of cytotoxic T lymphocytes. Nat. Immunol. 12, 352–361 (2011).
Cruz-Orcutt, N., Vacaflores, A., Connolly, S.F., Bunnell, S.C. & Houtman, J.C. Activated PLC-γ1 is catalytically induced at LAT but activated PLC-γ1 is localized at both LAT- and TCR-containing complexes. Cell. Signal. 26, 797–805 (2014).
Zhang, W. et al. Essential role of LAT in T cell development. Immunity 10, 323–332 (1999).
Aguado, E. et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296, 2036–2040 (2002).
Sommers, C.L. et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296, 2040–2043 (2002).
Chuck, M.I., Zhu, M., Shen, S. & Zhang, W. The role of the LAT-PLC-γ1 interaction in T regulatory cell function. J. Immunol. 184, 2476–2486 (2010).
Koonpaew, S., Shen, S., Flowers, L. & Zhang, W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J. Exp. Med. 203, 119–129 (2006).
Wang, Y. et al. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J. Immunol. 180, 1565–1575 (2008).
Genton, C. et al. The Th2 lymphoproliferation developing in LatY136F mutant mice triggers polyclonal B cell activation and systemic autoimmunity. J. Immunol. 177, 2285–2293 (2006).
Chevrier, S., Genton, C., Malissen, B., Malissen, M. & Acha-Orbea, H. Dominant role of CD80–CD86 Over CD40 and ICOSL in the massive polyclonal B cell activation mediated by LAT(Y136F) CD4+ T cells. Front Immunol 3, 27 (2012).
Nuñez-Cruz, S. et al. LAT regulates gammadelta T cell homeostasis and differentiation. Nat. Immunol. 4, 999–1008 (2003).
Archambaud, C. et al. STAT6 deletion converts the Th2 inflammatory pathology afflicting Lat(Y136F) mice into a lymphoproliferative disorder involving Th1 and CD8 effector T cells. J. Immunol. 182, 2680–2689 (2009).
Roncagalli, R., Mingueneau, M., Gregoire, C., Malissen, M. & Malissen, B. LAT signaling pathology: an “autoimmune” condition without T cell self-reactivity. Trends Immunol. 31, 253–259 (2010).
Kortum, R.L. et al. A phospholipase C-γ1-independent, RasGRP1-ERK-dependent pathway drives lymphoproliferative disease in linker for activation of T cells-Y136F mutant mice. J. Immunol. 190, 147–158 (2013).
Cao, L. et al. Quantitative phosphoproteomics reveals SLP-76 dependent regulation of PAG and Src family kinases in T cells. PLoS ONE 7, e46725 (2012).
Siggs, O.M. et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity 27, 912–926 (2007).
Hirota, K. et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47 (2007).
Hsu, L.Y., Tan, Y.X., Xiao, Z., Malissen, M. & Weiss, A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J. Exp. Med. 206, 2527–2541 (2009).
Vang, T. et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 37, 1317–1319 (2005).
Oh-Hora, M. et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 9, 432–443 (2008).
Chabod, M. et al. A spontaneous mutation of the rat Themis gene leads to impaired function of regulatory T cells linked to inflammatory bowel disease. PLoS Genet. 8, e1002461 (2012).
McDonald, C.B. et al. Multivalent binding and facilitated diffusion account for the formation of the Grb2-Sos1 signaling complex in a cooperative manner. Biochemistry 51, 2122–2135 (2012).
Houtman, J.C. et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat. Struct. Mol. Biol. 13, 798–805 (2006).One of three key papers (refs. 57, 58 and 99) demonstrating the role of cooperative interactions in the assembly of the TCR signal-transduction network.
Kortum, R.L. et al. The ability of Sos1 to oligomerize the adaptor protein LAT is separable from its guanine nucleotide exchange activity in vivo. Sci. Signal. 6, ra99 (2013).
Sherman, E. et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity 35, 705–720 (2011).One of two key papers (refs. 59 and 61) describing the use of super-resolution microscopy to determine the nanoscale organisation of signaling molecules in T cells.
Sherman, E., Barr, V.A. & Samelson, L.E. Resolving multi-molecular protein interactions by photoactivated localization microscopy. Methods 59, 261–269 (2013).
Hsu, C.J. et al. Ligand mobility modulates immunological synapse formation and T cell activation. PLoS ONE 7, e32398 (2012).
James, J.R. et al. The T cell receptor triggering apparatus is composed of monovalent or monomeric proteins. J. Biol. Chem. 286, 31993–32001 (2011).
Roybal, K.T., Sinai, P., Verkade, P., Murphy, R.F. & Wulfing, C. The actin-driven spatiotemporal organization of T-cell signaling at the system scale. Immunol. Rev. 256, 133–147 (2013).
Bonello, G. et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J. Cell Sci. 117, 1009–1016 (2004).
Williamson, D.J. et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat. Immunol. 12, 655–662 (2011).One of three key papers (refs. 65–67) suggesting a role for intracellular vesicular Lat molecules in the TCR signal-transduction network.
Larghi, P. et al. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat. Immunol. 14, 723–731 (2013).
Purbhoo, M.A. et al. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci. Signal. 3, ra36 (2010).
Malissen, B. & Marguet, D. La(s)t but not least. Nat. Immunol. 12, 592–593 (2011).
Balagopalan, L., Barr, V.A., Kortum, R.L., Park, A.K. & Samelson, L.E. Cutting edge: cell surface linker for activation of T cells is recruited to microclusters and is active in signaling. J. Immunol. 190, 3849–3853 (2013).
Soares, H. et al. Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J. Exp. Med. 210, 2415–2433 (2013).Key paper suggesting that intracellular vesicles fuse with the plasma membrane beyond the IS to generate signaling 'nanoterritories'.
Lillemeier, B.F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 11, 90–96 (2010).
Choquet, D. & Triller, A. The dynamic synapse. Neuron 80, 691–703 (2013).
Vardhana, S., Choudhuri, K., Varma, R. & Dustin, M.L. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity 32, 531–540 (2010).
Lasserre, R. et al. Release of serine/threonine-phosphorylated adaptors from signaling microclusters down-regulates T cell activation. J. Cell Biol. 195, 839–853 (2011).
Choudhuri, K. et al. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 507, 118–123 (2014).
Feinerman, O., Veiga, J., Dorfman, J.R., Germain, R.N. & Altan-Bonnet, G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science 321, 1081–1084 (2008).
Toettcher, J.E., Weiner, O.D. & Lim, W.A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).Key paper demonstrating the power of using cellular optogenetics to delineate a complex signal-transduction network.
Nakakuki, T. et al. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell 141, 884–896 (2010).
Breart, B., Lemaitre, F., Celli, S. & Bousso, P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J. Clin. Invest. 118, 1390–1397 (2008).
Soudja, S.M., Ruiz, A.L., Marie, J.C. & Lauvau, G. Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562 (2012).
Acuto, O., Di Bartolo, V. & Michel, F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 8, 699–712 (2008).
Malissen, B. An evolutionary and structural perspective on T cell antigen receptor function. Immunol. Rev. 191, 7–27 (2003).
Pauker, M.H., Reicher, B., Fried, S., Perl, O. & Barda-Saad, M. Functional cooperation between the proteins Nck and ADAP is fundamental for actin reorganization. Mol. Cell. Biol. 31, 2653–2666 (2011).
Ophir, M.J., Liu, B.C. & Bunnell, S.C. The N terminus of SKAP55 enables T cell adhesion to TCR and integrin ligands via distinct mechanisms. J. Cell Biol. 203, 1021–1041 (2013).
Balagopalan, L., Coussens, N.P., Sherman, E., Samelson, L.E. & Sommers, C.L. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb. Perspect. Biol. 2, a005512 (2010).
Gomez, T.S. et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat. Immunol. 6, 261–270 (2005).
Jun, J.E., Rubio, I. & Roose, J.P. Regulation of Ras exchange factors and cellular localization of Ras activation by lipid messengers in T cells. Front Immunol 4, 239 (2013).
Le Floc'h, A. et al. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J. Exp. Med. 210, 2721–2737 (2013).
Shui, J.W. et al. Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses. Nat. Immunol. 8, 84–91 (2007).
Di Bartolo, V. et al. A novel pathway down-modulating T cell activation involves HPK-1-dependent recruitment of 14–3-3 proteins on SLP-76. J. Exp. Med. 204, 681–691 (2007).
San Luis, B., Sondgeroth, B., Nassar, N. & Carpino, N. Sts-2 is a phosphatase that negatively regulates ζ-associated protein (ZAP)-70 and T cell receptor signaling pathways. J. Biol. Chem. 286, 15943–15954 (2011).
Balagopalan, L. et al. Enhanced T-cell signaling in cells bearing linker for activation of T-cell (LAT) molecules resistant to ubiquitylation. Proc. Natl. Acad. Sci. USA 108, 2885–2890 (2011).
Fu, G. et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 504, 441–445 (2013).
Paster, W. et al. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J. Immunol. 190, 3749–3756 (2013).
Lesourne, R. et al. Themis, a T cell-specific protein important for late thymocyte development. Nat. Immunol. 10, 840–847 (2009).
Dong, S. et al. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J. Exp. Med. 203, 2509–2518 (2006).
Yasuda, T. et al. Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int. Immunol. 19, 487–495 (2007).
Schoenborn, J.R., Tan, Y.X., Zhang, C., Shokat, K.M. & Weiss, A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci. Signal. 4, ra59 (2011).
Coussens, N.P. et al. Multipoint binding of the SLP-76 SH2 domain to ADAP is critical for oligomerization of SLP-76 signaling complexes in stimulated T cells. Mol. Cell. Biol. 33, 4140–4151 (2013).
Bunnell, S.C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Douglass, A.D. & Vale, R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950 (2005).
Kleiman, L.B., Maiwald, T., Conzelmann, H., Lauffenburger, D.A. & Sorger, P.K. Rapid phospho-turnover by receptor tyrosine kinases impacts downstream signaling and drug binding. Mol. Cell 43, 723–737 (2011).
Yokosuka, T. et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity 29, 589–601 (2008).
Dustin, M.L. The cellular context of T cell signaling. Immunity 30, 482–492 (2009).
Acknowledgements
This paper is dedicated to the memory of François Kourilsky. We thank R. Germain and P. Bongrand for discussions. Supported by the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Aix-Marseille Université, French National Infrastructure for Mouse Phenogenomics (PHENOMIN), Agence Nationale de Recherche (Basilic project to M.M.) and European Research Council (“Integrate” grant to B.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Malissen, B., Grégoire, C., Malissen, M. et al. Integrative biology of T cell activation. Nat Immunol 15, 790–797 (2014). https://doi.org/10.1038/ni.2959
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2959
This article is cited by
-
Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders
Molecular Neurodegeneration (2020)
-
Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response
Nature Communications (2019)
-
Interference with KCTD9 inhibits NK cell activation and ameliorates fulminant liver failure in mice
BMC Immunology (2018)
-
SYK expression in monomorphic epitheliotropic intestinal T-cell lymphoma
Modern Pathology (2018)
-
Vanadium in Biosphere and Its Role in Biological Processes
Biological Trace Element Research (2018)