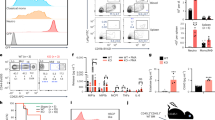
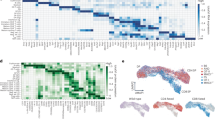
Abstract
The differentiation of hematopoietic stem cells into cells of the immune system has been studied extensively in mammals, but the transcriptional circuitry that controls it is still only partially understood. Here, the Immunological Genome Project gene-expression profiles across mouse immune lineages allowed us to systematically analyze these circuits. To analyze this data set we developed Ontogenet, an algorithm for reconstructing lineage-specific regulation from gene-expression profiles across lineages. Using Ontogenet, we found differentiation stage–specific regulators of mouse hematopoiesis and identified many known hematopoietic regulators and 175 previously unknown candidate regulators, as well as their target genes and the cell types in which they act. Among the previously unknown regulators, we emphasize the role of ETV5 in the differentiation of γδ T cells. As the transcriptional programs of human and mouse cells are highly conserved, it is likely that many lessons learned from the mouse model apply to humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Heng, T.S.P. et al. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Iwasaki, H. & Akashi, K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity 26, 726–740 (2007).
Novershtern, N. et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144, 296–309 (2011).
Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl. Acad. Sci. USA 110, 2946–2951 (2013).
Kim, H.D., Shay, T., O'Shea, E.K. & Regev, A. Transcriptional regulatory circuits: predicting numbers from alphabets. Science 325, 429–432 (2009).
Lee, S.-I. et al. Learning a prior on regulatory potential from eQTL data. PLoS Genet. 5, e1000358 (2009).
Segal, E. et al. Module networks: identifying regulatory modules and their condition-specific regulators from gene expression data. Nat. Genet. 34, 166–176 (2003).
Capaldi, A.P. et al. Structure and function of a transcriptional network activated by the MAPK Hog1. Nat. Genet. 40, 1300–1306 (2008).
Ram, O. et al. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell 147, 1628–1639 (2011).
Miller, J.C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Zou, H. & Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Series B Stat. Methodol. 67, 301–320 (2005).
Tamura, T. et al. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 174, 2573–2581 (2005).
Orkin, S.H. & Zon, L.I. SnapShot: hematopoiesis. Cell 132, 712.e711–712.e712 (2008).
Pierre, M., Yoshimoto, M., Huang, L., Richardson, M. & Yoder, M.C. VEGF and IHH rescue definitive hematopoiesis in Gata-4 and Gata-6–deficient murine embryoid bodies. Exp. Hematol. 37, 1038–1053 (2009).
Kishi, H. et al. Lineage-specific regulation of the murine RAG-2 promoter: GATA-3 in T cells and Pax-5 in B cells. Blood 95, 3845–3852 (2000).
Bodor, J., Fehervari, Z., Diamond, B. & Sakaguchi, S. ICER/CREM-mediated transcriptional attenuation of IL-2 and its role in suppression by regulatory T cells. Eur. J. Immunol. 37, 884–895 (2007).
Ji, H. et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 467, 338–342 (2010).
Lee, J.-W. et al. DACH1 regulates cell cycle progression of myeloid cells through the control of cyclin D, Cdk 4/6 and p21Cip1. Biochem. Biophys. Res. Commun. 420, 91–95 (2012).
Ng, S.S.M., Chang, T.-H., Tailor, P., Ozato, K. & Kino, T. Virus-induced differential expression of nuclear receptors and coregulators in dendritic cells: Implication to interferon production. FEBS Lett. 585, 1331–1337 (2011).
Wang, T. et al. Inhibition of activation-induced death of dendritic cells and enhancement of vaccine efficacy via blockade of MINOR. Blood 113, 2906–2913 (2009).
Neumann, M. et al. Differential expression of Rel/NF-κB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood 95, 277–285 (2000).
Outram, S.V. et al. KLF13 influences multiple stages of both B and T cell development. Cell Cycle 7, 2047–2055 (2008).
Yamada, T., Park, C.S., Mamonkin, M. & Lacorazza, H.D. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Kruppel-like factors KLF4 and KLF2. Nat. Immunol. 10, 618–626 (2009).
Narayan, K. et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat. Immunol. 13, 511–518 (2012).
Yosef, N. & Regev, A. Impulse control: temporal dynamics in gene transcription. Cell 144, 886–896 (2011).
Bendall, S.C. et al. Single-cell Mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Blatt, M., Wiseman, S. & Domany, E. Superparamagnetic clustering of data. Phys. Rev. Lett. 76, 3251–3254 (1996).
Frey, B.J. & Dueck, D. Clustering by passing messages between data points. Science 315, 972–976 (2007).
Matys, V. et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 34, D108–D110 (2006).
Sandelin, A., Alkema, W., Engström, P., Wasserman, W.W. & Lenhard, B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 32 (suppl. 1), D91–D94 (2004).
Badis, G. et al. Diversity and complexity in DNA recognition by transcription factors. Science 324, 1720–1723 (2009).
Berger, M.F. et al. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133, 1266–1276 (2008).
Tibshirani, R., Saunders, M., Rosset, S., Zhu, J. & Knight, K. Sparsity and smoothness via the fused lasso. J. R. Stat. Soc. Series B Stat. Methodol. 67, 91–108 (2005).
Boyd, S.P. & Vandenberghe, L. in Convex Optimization, Ch 11.7 (Cambridge University, Cambridge, UK, 2004).
Vilella, A.J. et al. EnsemblCompara GeneTrees: complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 19, 327–335 (2009).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Zhang, Z., Verheyden, J.M., Hassell, J.A. & Sun, X. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev. Cell 16, 607–613 (2009).
Acknowledgements
We thank the ImmGen core team (including M. Painter and S. Davis) for help with data generation and processing; eBioscience, Affymetrix and Expression Analysis for support of ImmGen; L. Gaffney for help with figure preparation and layout of the lineage tree; S. Hart for initial layout of the lineage tree; and A. Liberzon (Molecular Signatures Database) for the positional gene sets for mouse. Supported by National Institute of Allergy and Infectious Diseases (R24 AI072073 to the ImmGen Consortium), the US National Institutes of Health (A.R.; and U54-CA149145 and 149644.0103 to V.J. and D.K.), the Burroughs Wellcome Fund (A.R.), the Klarman Cell Observatory (A.R.), the Howard Hughes Medical Institute (A.R.), the Merkin Foundation for Stem Cell Research at the Broad Institute (A.R.) and the National Science Foundation (DBI-0345474 to V.J. and D.K.).
Author information
Authors and Affiliations
Consortia
Contributions
V.J., T.S., A.R. and D.K. designed the study; V.J. developed the algorithm, with input from T.S., A.R. and D.K.; T.S. analyzed the data; K.S. did experiments; O.Z. provided the motif-related code and participated in writing; X.S. generated a mouse model; J.K. designed the experiments and participated in writing the manuscript; and V.J., T.S., A.R. and D.K. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members and affiliations appears at the end of the paper.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Notes 1–3 (PDF 5891 kb)
Supplementary Table 1
Supplementary Table 1 (XLS 209 kb)
Supplementary Table 2
Supplementary Table 2 (XLS 1733 kb)
Supplementary Table 3
Supplementary Table 3 (XLSX 41 kb)
Supplementary Table 4
Supplementary Table 4 (XLSX 34 kb)
Supplementary Table 5
Supplementary Table 5 (XLS 181 kb)
Supplementary Table 6
Supplementary Table 6 (XLS 200 kb)
Supplementary Table 7
Supplementary Table 7 (XLS 712 kb)
Supplementary Table 8
Supplementary Table 8 (XLS 36 kb)
Supplementary Table 9
Supplementary Table 9 (XLS 203 kb)
Supplementary Table 10
Supplementary Table 10 (XLSX 56 kb)
Supplementary Table 11
Supplementary Table 11 (XLSX 28 kb)
Supplementary Table 12
Supplementary Table 12 (XLS 106 kb)
Supplementary Table 13
Supplementary Table 13 (XLSX 16 kb)
Rights and permissions
About this article
Cite this article
Jojic, V., Shay, T., Sylvia, K. et al. Identification of transcriptional regulators in the mouse immune system. Nat Immunol 14, 633–643 (2013). https://doi.org/10.1038/ni.2587
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2587
This article is cited by
-
E26 transformation-specific transcription variant 5 in development and cancer: modification, regulation and function
Journal of Biomedical Science (2023)
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
oCEM: Automatic detection and analysis of overlapping co-expressed gene modules
BMC Genomics (2022)
-
Interleukin-1 contributes to clonal expansion and progression of bone marrow fibrosis in JAK2V617F-induced myeloproliferative neoplasm
Nature Communications (2022)
-
A combination of intrinsic and extrinsic features improves prognostic prediction in malignant pleural mesothelioma
British Journal of Cancer (2022)