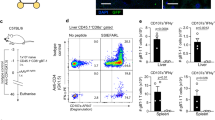
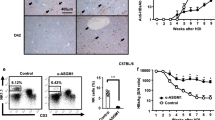
Abstract
Chronic infection is difficult to overcome because of exhaustion or depletion of cytotoxic effector CD8+ T cells (cytotoxic T lymphoytes (CTLs)). Here we report that signaling via Toll-like receptors (TLRs) induced intrahepatic aggregates of myeloid cells that enabled the population expansion of CTLs (iMATEs: 'intrahepatic myeloid-cell aggregates for T cell population expansion') without causing immunopathology. In the liver, CTL proliferation was restricted to iMATEs that were composed of inflammatory monocyte–derived CD11b+ cells. Signaling via tumor-necrosis factor (TNF) caused iMATE formation that facilitated costimulation dependent on the receptor OX40 for expansion of the CTL population. The iMATEs arose during acute viral infection but were absent during chronic viral infection, yet they were still induced by TLR signaling. Such hepatic expansion of the CTL population controlled chronic viral infection of the liver after vaccination with DNA. Thus, iMATEs are dynamic structures that overcome regulatory cues that limit the population expansion of CTLs during chronic infection and can be used in new therapeutic vaccination strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Crispe, I.N. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3, 51–62 (2003).
Thomson, A.W. & Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 10, 753–766 (2010).
Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Protzer, U., Maini, M.K. & Knolle, P.A. Living in the liver: hepatic infections. Nat. Rev. Immunol. 12, 201–213 (2012).
Isogawa, M., Furuichi, Y. & Chisari, F.V. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 23, 53–63 (2005).
Das, A. et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 205, 2111–2124 (2008).
Lopes, A.R. et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J. Clin. Invest. 118, 1835–1845 (2008).
Chakravarty, S. et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 13, 1035–1041 (2007).
Cockburn, I.A. et al. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 6, e1000877 (2010).
Wong, P. & Pamer, E.G. Feedback regulation of pathogen-specific T cell priming. Immunity 18, 499–511 (2003).
Kang, S.S. et al. Migration of cytotoxic lymphocytes in cell cycle permits local MHC I-dependent control of division at sites of viral infection. J. Exp. Med. 208, 747–759 (2011).
Neyt, K., Perros, F., GeurtsvanKessel, C.H., Hammad, H. & Lambrecht, B.N. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 33, 297–305 (2012).
Moyron-Quiroz, J.E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Rangel-Moreno, J. et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol. 12, 639–646 (2011).
Egen, J.G. et al. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271–284 (2008).
Egen, J.G. et al. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 34, 807–819 (2011).
Curtsinger, J.M., Johnson, C.M. & Mescher, M.F. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171, 5165–5171 (2003).
Lee, S.W., Park, Y., Yoo, J.K., Choi, S.Y. & Sung, Y.C. Inhibition of TCR-induced CD8 T cell death by IL-12: regulation of Fas ligand and cellular FLIP expression and caspase activation by IL-12. J. Immunol. 170, 2456–2460 (2003).
Macgregor, J.N. et al. Ex vivo culture with interleukin (IL)-12 improves CD8+ T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-γ but requires perforin. Cancer Res. 66, 4913–4921 (2006).
Gommerman, J.L. & Browning, J.L. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat. Rev. Immunol. 3, 642–655 (2003).
Drayton, D.L., Liao, S., Mounzer, R.H. & Ruddle, N.H. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7, 344–353 (2006).
Serbina, N.V., Salazar-Mather, T.P., Biron, C.A., Kuziel, W.A. & Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Mescher, M.F. et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211, 81–92 (2006).
Croft, M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 (2010).
Stabenow, D. et al. Bioluminescence imaging allows measuring CD8 T cell function in the liver. Hepatology 51, 1430–1437 (2010).
Huang, L.R. et al. Transfer of HBV genomes using low doses of adenovirus vectors leads to persistent infection in immune competent mice. Gastroenterology 142, 1447–1450 (2012).
Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12, 352–366 (2012).
Peters, A. et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35, 986–996 (2011).
Hochweller, K. et al. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc. Natl. Acad. Sci. USA 107, 5931–5936 (2010).
Sacher, T. et al. CpG-ODN-induced inflammation is sufficient to cause T-cell-mediated autoaggression against hepatocytes. Eur. J. Immunol. 32, 3628–3637 (2002).
Lang, K.S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 11, 138–145 (2005).
Lang, K.S. et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 116, 2456–2463 (2006).
Hohl, T.M. et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6, 470–481 (2009).
Wohlleber, D. et al. TNF-induced target cell killing by CTL activated through cross-presentation. Cell Reports 2, 478–487 (2012).
Haybaeck, J. et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 16, 295–308 (2009).
Wolf, M.J. et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 22, 91–105 (2012).
Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132, 2487–2500 (2009).
Jenne, C.N., Wong, C.H., Petri, B. & Kubes, P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS ONE 6, e25109 (2011).
Acknowledgements
We thank D. Kull, R. Hillermann, O. Seelbach, N. Simonavicius, M. Wolf and the flow cytometry core facility of the Institutes of Molecular Medicine and Experimental Immunology, University of Bonn for technical support. Supported by the Helmholtz Association (M.H.), the Hofschneider Foundation (M.H.), the European Research Council (LiverCancerMech; M.H.), the Helmholtz Alliance Preclinical Comprehensive Cancer Center (M.H.), Sonderforschungsbereich (36 to M.H., and 704, TR57 and 670 to P.K.), the Helmholtz Alliance on Immunotherapy of Cancer (P.K.), the Bundesministerium für Bildung und Forschung projects Virtual Liver and PeTra (P.K.) and the Excellence Cluster ImmunoSensation (P.K.).
Author information
Authors and Affiliations
Contributions
L.-R.H., D.W., F.R., R.-L.C., Z.A. and F.A.S. did the experiments; C.N.J. and P.K. designed and did intravital microscopy experiments; M.O. and H.-P.D. did electron microscopy studies; N.v.R., E.S., N.G. and M.C. contributed reagents and contributed to experimental design; C.K., U.P., M.H. and P.A.K. designed experiments; and L.-R.H., M.H. and P.A.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 3452 kb)
Supplementary Video 1
Hepatic microvasculature and uptake of TLR9-L by sinusoidal cells. (MOV 3412 kb)
Supplementary Video 2
Hepatic microvasculature and uptake of TLR9-L by sinusoidal cells. (MOV 4486 kb)
Supplementary Video 3
Circulating CD11b+ cells adhere in sinusoids after TLR9-L application. (MOV 6787 kb)
Supplementary Video 4
Circulating CD11b+ cells adhere in sinusoids after TLR9-L application. (MOV 4230 kb)
Supplementary Video 5
CD11b+ cells form non-perfused structures, iMATEs. (MOV 6593 kb)
Supplementary Video 6
CD11b+ cells form non-perfused structures, iMATEs. (MOV 5457 kb)
Supplementary Video 7
CD11b+ cells form non-perfused structures, iMATEs. (MOV 1295 kb)
Supplementary Video 8
CD11b+ cells form non-perfused structures, iMATEs. (MOV 4833 kb)
Supplementary Video 9
CD8+ T cells migrate slowly within iMATEs. (MOV 11059 kb)
Supplementary Video 10
3D structures of iMATEs. (MOV 3268 kb)
Supplementary Video 11
3D structures of iMATEs. (MOV 3577 kb)
Supplementary Video 12
3D structures of iMATEs. (MOV 5917 kb)
Rights and permissions
About this article
Cite this article
Huang, LR., Wohlleber, D., Reisinger, F. et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8+ T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 14, 574–583 (2013). https://doi.org/10.1038/ni.2573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2573
This article is cited by
-
Contribution of T- and B-cell intrinsic toll-like receptors to the adaptive immune response in viral infectious diseases
Cellular and Molecular Life Sciences (2022)
-
Harnessing the liver to induce antigen-specific immune tolerance
Seminars in Immunopathology (2022)
-
HBV X protein-based therapeutic vaccine accelerates viral antigen clearance by mobilizing monocyte infiltration into the liver in HBV carrier mice
Journal of Biomedical Science (2020)
-
Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal
Nature Immunology (2020)
-
Tissue-infiltrating lymphocytes signature predicts survival in patients with early/intermediate stage hepatocellular carcinoma
BMC Medicine (2019)