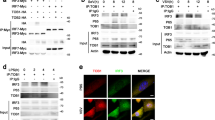
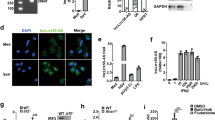
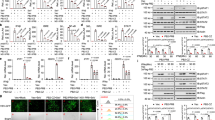
Abstract
The production of type I interferon is essential for viral clearance but is kept under tight control to avoid unnecessary tissue damage from hyperinflammatory responses. Here we found that OASL1 inhibited translation of IRF7, the master transcription factor for type I interferon, and thus negatively regulated the robust production of type I interferon during viral infection. OASL1 inhibited the translation of IRF7 mRNA by binding to the 5′ untranslated region (UTR) of IRF7 and possibly by inhibiting scanning of the 43S preinitiation complex along the message. Oasl1−/− mice were resistant to viral infection because of the greater abundance of type I interferon, which suggests that OASL1 could be a potential therapeutic target for boosting the production of type I interferon during viral infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
GenBank/EMBL/DDBJ
Change history
22 April 2013
In the version of this article initially published, the second affiliation for Young-Joon Kim was missing. The correct affiliation includes the following: Department of Integrated Omics for Biomedical Science, WCU Program of Graduate School, Yonsei University, Seoul, Republic of Korea. The error has been corrected in the HTML and PDF versions of the article.
References
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Lee, M.S. & Kim, Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76, 447–480 (2007).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Sato, M. et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13, 539–548 (2000).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Honda, K., Takaoka, A. & Taniguchi, T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Wathelet, M.G. et al. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1, 507–518 (1998).
Ning, S., Pagano, J.S. & Barber, G.N. IRF7: activation, regulation, modification and function. Genes Immun. 12, 399–414 (2011).
Sato, M. et al. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441, 106–110 (1998).
Stark, G.R., Kerr, I.M., Williams, B.R., Silverman, R.H. & Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Silverman, R.H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 81, 12720–12729 (2007).
Kakuta, S., Shibata, S. & Iwakura, Y. Genomic structure of the mouse 2′,5′-oligoadenylate synthetase gene family. J. Interferon Cytokine Res. 22, 981–993 (2002).
Kristiansen, H., Gad, H.H., Eskildsen-Larsen, S., Despres, P. & Hartmann, R. The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities. J. Interferon Cytokine Res. 31, 41–47 (2011).
Yan, W. et al. Mice deficient in oocyte-specific oligoadenylate synthetase-like protein OAS1D display reduced fertility. Mol. Cell Biol. 25, 4615–4624 (2005).
Mashimo, T. et al. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. USA 99, 11311–11316 (2002).
Eskildsen, S., Justesen, J., Schierup, M.H. & Hartmann, R. Characterization of the 2′-5′-oligoadenylate synthetase ubiquitin-like family. Nucleic Acids Res. 31, 3166–3173 (2003).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Gitlin, L. et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103, 8459–8464 (2006).
Jung, A. et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J. Virol. 82, 196–206 (2008).
Bonnefoy, F. et al. Plasmacytoid dendritic cells play a major role in apoptotic leukocyte-induced immune modulation. J. Immunol. 186, 5696–5705 (2011).
Rose, S., Misharin, A. & Perlman, H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 81, 343–350 (2012).
Kadeppagari, R.K., Sanchez, R.L. & Foster, T.P. HSV-2 inhibits type-I interferon signaling via multiple complementary and compensatory STAT2-associated mechanisms. Virus Res. 167, 273–284 (2012).
Herdy, B. et al. Translational control of the activation of transcription factor NF-κB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat. Immunol. 13, 543–550 (2012).
Bachand, F. & Silver, P.A. PRMT3 is a ribosomal protein methyltransferase that affects the cellular levels of ribosomal subunits. EMBO J. 23, 2641–2650 (2004).
Sonenberg, N. & Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009).
Komar, A.A. & Hatzoglou, M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle 10, 229–240 (2011).
Schoggins, J.W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Hiramatsu, N., Kasai, A., Hayakawa, K., Yao, J. & Kitamura, M. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res. 34, e93 (2006).
Gruber, A.R., Lorenz, R., Bernhart, S.H., Neubock, R. & Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 36, W70–W74 (2008).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Barbalat, R., Ewald, S.E., Mouchess, M.L. & Barton, G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 29, 185–214 (2011).
Hartmann, R., Justesen, J., Sarkar, S.N., Sen, G.C. & Yee, V.C. Crystal structure of the 2′-specific and double-stranded RNA-activated interferon-induced antiviral protein 2′-5′-oligoadenylate synthetase. Mol. Cell 12, 1173–1185 (2003).
Wang, L., Jeng, K.S. & Lai, M.M. Poly(C)-binding protein 2 interacts with sequences required for viral replication in the hepatitis C virus (HCV) 5′ untranslated region and directs HCV RNA replication through circularizing the viral genome. J. Virol. 85, 7954–7964 (2011).
Muckenthaler, M.U., Galy, B. & Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 28, 197–213 (2008).
Richards, K.H. & Macdonald, A. Putting the brakes on the anti-viral response: negative regulators of type I interferon (IFN) production. Microbes Infect. 13, 291–302 (2011).
Saitoh, T. et al. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat. Immunol. 7, 598–605 (2006).
Higgs, R. et al. Self protection from anti-viral responses–Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral Toll-Like receptors. PLoS ONE 5, e11776 (2010).
Colina, R. et al. Translational control of the innate immune response through IRF-7. Nature 452, 323–328 (2008).
Marques, J. et al. The p59 oligoadenylate synthetase-like protein possesses antiviral activity that requires the C-terminal ubiquitin-like domain. J. Gen. Virol. 89, 2767–2772 (2008).
Perelygin, A.A., Zharkikh, A.A., Scherbik, S.V. & Brinton, M.A. The mammalian 2′-5′ oligoadenylate synthetase gene family: evidence for concerted evolution of paralogous Oas1 genes in Rodentia and Artiodactyla. J. Mol. Evol. 63, 562–576 (2006).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Naik, S.H. et al. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174, 6592–6597 (2005).
Conner, D.A. Mouse embryo fibroblast (MEF) feeder cell preparation. Curr. Protoc. Mol. Biol. 51, 23.2.1–23.2.7 (2001).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Samarajiwa, S.A., Forster, S., Auchettl, K. & Hertzog, P.J. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 37, D852–D857 (2009).
Smith, D.E. & Fisher, P.A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J. Cell Biol. 99, 20–28 (1984).
Morin, B. et al. High yield synthesis, purification and characterisation of the RNase L activators 5′-triphosphate 2′-5′-oligoadenylates. Antiviral Res. 87, 345–352 (2010).
Acknowledgements
We thank S. Park and Y. Seo for technical support in the microinjection of embryonic stem cells; J. Ahn and H. Jung for technical help in mouse maintenance; J. Kang and Q. Zhang for technical help in the molecular studies; and G. Dranoff (Harvard Medical School) for the melanoma cell lines B16-FLT3L and B16-GM-CSF. Supported by the Global Research Laboratory program of the Ministry of Education, Science and Technology of Korea (MEST; K20704000006-10A0500-00610 to Y.-J.K.), the World Class University program funded by the Korean government (MEST) through the National Research Foundation of Korea (R312010000100860 to Y.-J.K.), the National Research Foundation of Korea funded by the Korean government (MEST; 2012028272 to Y.-J.K.) and the Korea Genetically Engineered Mouse Center Program of the National Research Foundation of the Korean government (MEST; 2011-0020603 to G.T.O.).
Author information
Authors and Affiliations
Contributions
M.S.L. and Y.-J.K. designed the project; M.S.L. and B.K. did the experiments; M.S.L., B.K. and Y.-J.K. wrote the paper; and G.T.O. managed the generation of the Oasl1-deficient mice from targeted embryonic stem cells.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Tables 1–4 (PDF 1374 kb)
Rights and permissions
About this article
Cite this article
Lee, M., Kim, B., Oh, G. et al. OASL1 inhibits translation of the type I interferon–regulating transcription factor IRF7. Nat Immunol 14, 346–355 (2013). https://doi.org/10.1038/ni.2535
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2535
This article is cited by
-
Interferons as negative regulators of ILC2s in allergic lung inflammation and respiratory viral infections
Journal of Molecular Medicine (2023)
-
Cellular origins of dsRNA, their recognition and consequences
Nature Reviews Molecular Cell Biology (2022)
-
2′–5′ oligoadenylate synthetase‑like 1 (OASL1) protects against atherosclerosis by maintaining endothelial nitric oxide synthase mRNA stability
Nature Communications (2022)
-
Functional divergence of oligoadenylate synthetase 1 (OAS1) proteins in Tetrapods
Science China Life Sciences (2022)
-
Type I Interferons in the Pathogenesis and Treatment of Autoimmune Diseases
Clinical Reviews in Allergy & Immunology (2020)