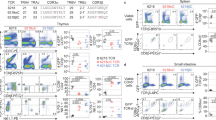
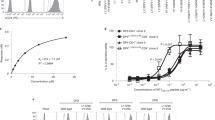
Abstract
The binding of T cell antigen receptors (TCRs) to specific complexes of peptide and major histocompatibility complex (pMHC) is typically of very low affinity, which necessitates the use of multimeric pMHC complexes to label T lymphocytes stably. We report here the development of pMHC complexes able to be crosslinked by ultraviolet irradiation; even as monomers, these efficiently and specifically stained cognate T cells. We also used this reagent to probe T cell activation and found that a covalently bound pMHC was more stimulatory than an agonist pMHC on lipid bilayers. This finding suggested that serial engagement of TCRs is dispensable for activation when a substantial fraction of TCRs are stably engaged. Finally, pMHC-bound TCRs were 'preferentially' transported into the central supramolecular activation cluster after activation, which suggested that ligand engagement enabled linkage of the TCR and its associated CD3 signaling molecules to the cytoskeleton.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davis, M.M. et al. T cells as a self-referential, sensory organ. Annu. Rev. Immunol. 25, 681–695 (2007).
Altman, J.D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Davis, M.M., Altman, J.D. & Newell, E.W. Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Nat. Rev. Immunol. 11, 551–558 (2011).
McKeithan, T.W. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl. Acad. Sci. USA 92, 5042–5046 (1995).
Valitutti, S., Muller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 375, 148–151 (1995).
Kalergis, A.M. et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat. Immunol. 2, 229–234 (2001).
Holler, P.D. & Kranz, D.M. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18, 255–264 (2003).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Huppa, J.B. et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature 463, 963–967 (2010).
González, P.A. et al. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. Proc. Natl. Acad. Sci. USA 102, 4824–4829 (2005).
Thomas, S. et al. Human T cells expressing affinity-matured TCR display accelerated responses but fail to recognize low density of MHC-peptide antigen. Blood 118, 319–329 (2011).
Irving, M. et al. Interplay between T cell receptor binding kinetics and the level of cognate peptide presented by major histocompatibility complexes governs CD8+ T cell responsiveness. J. Biol. Chem. published online, doi:10.1074/jbc.M112.357673 (1 May 2012).
Monks, C.R., Freiberg, B.A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Wülfing, C., Sjaastad, M.D. & Davis, M.M. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl. Acad. Sci. USA 95, 6302–6307 (1998).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Rozdzial, M.M., Malissen, B. & Finkel, T.H. Tyrosine-phosphorylated T cell receptor zeta chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity 3, 623–633 (1995).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Campi, G., Varma, R. & Dustin, M.L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202, 1031–1036 (2005).
Billadeau, D.D., Nolz, J.C. & Gomez, T.S. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 7, 131–143 (2007).
Burkhardt, J.K., Carrizosa, E. & Shaffer, M.H. The actin cytoskeleton in T cell activation. Annu. Rev. Immunol. 26, 233–259 (2008).
Ilani, T., Vasiliver-Shamis, G., Vardhana, S., Bretscher, A. & Dustin, M.L. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat. Immunol. 10, 531–539 (2009).
Hashimoto-Tane, A. et al. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity 34, 919–931 (2011).
Altman, J.D., Reay, P.A. & Davis, M.M. Formation of functional peptide complexes of class II major histocompatibility complex proteins from subunits produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 90, 10330–10334 (1993).
Toebes, M. et al. Design and use of conditional MHC class I ligands. Nat. Med. 12, 246–251 (2006).
Grotenbreg, G.M. et al. Empty class II major histocompatibility complex created by peptide photolysis establishes the role of DM in peptide association. J. Biol. Chem. 282, 21425–21436 (2007).
Weber, K.S. et al. Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc. Natl. Acad. Sci. USA (in the press).
Rock, E.P. Structure-Function Analysis of Antigen-Specific T Cell Receptors Ph.D. thesis, Stanford Univ. 60–91, (1993).
Kaye, J. et al. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature 341, 746–749 (1989).
Cemerski, S. et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity 26, 345–355 (2007).
Newell, E.W. et al. Structural basis of specificity and cross-reactivity in T cell receptors specific for cytochrome c-I-Ek. J. Immunol. 186, 5823–5832 (2011).
Carbone, F.R. & Bevan, M.J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J. Exp. Med. 169, 603–612 (1989).
Fremont, D.H., Stura, E.A., Matsumura, M., Peterson, P.A. & Wilson, I.A. Crystal structure of an H-2Kb-ovalbumin peptide complex reveals the interplay of primary and secondary anchor positions in the major histocompatibility complex binding groove. Proc. Natl. Acad. Sci. USA 92, 2479–2483 (1995).
Purbhoo, M.A., Irvine, D.J., Huppa, J.B. & Davis, M.M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Juang, J. et al. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J. Exp. Med. 207, 1223–1234 (2010).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Boniface, J.J. et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity 9, 459–466 (1998).
Cochran, J.R., Cameron, T.O. & Stern, L.J. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity 12, 241–250 (2000).
Cebecauer, M. et al. CD8+ cytotoxic T lymphocyte activation by soluble major histocompatibility complex-peptide dimers. J. Biol. Chem. 280, 23820–23828 (2005).
Kim, S.T. et al. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 284, 31028–31037 (2009).
Li, Y.C. et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J. Immunol. 184, 5959–5963 (2010).
Ma, Z. & Finkel, T.H. T cell receptor triggering by force. Trends Immunol. 31, 1–6 (2010).
Howarth, M. et al. A monovalent streptavidin with a single femtomolar biotin binding site. Nat. Methods 3, 267–273 (2006).
Luescher, I.F., Cerottini, J.C. & Romero, P. Photoaffinity labeling of the T cell receptor on cloned cytotoxic T lymphocytes by covalent photoreactive ligand. J. Biol. Chem. 269, 5574–5582 (1994).
Luescher, I.F. et al. Structural analysis of TCR-ligand interactions studied on H-2Kd-restricted cloned CTL specific for a photoreactive peptide derivative. Immunity 3, 51–63 (1995).
Naeher, D. et al. A constant affinity threshold for T cell tolerance. J. Exp. Med. 204, 2553–2559 (2007).
Hudrisier, D. et al. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J. Immunol. 161, 553–562 (1998).
Leyva, E., Platz, M.S., Persy, G. & Wirz, J. Photochemistry of phenyl azide - the role of singlet and triplet phenylnitrene as transient intermediates. J. Am. Chem. Soc. 108, 3783–3790 (1986).
Varma, R., Campi, G., Yokosuka, T., Saito, T. & Dustin, M.L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25, 117–127 (2006).
Yokosuka, T. et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 6, 1253–1262 (2005).
Lee, K.H. et al. The immunological synapse balances T cell receptor signaling and degradation. Science 302, 1218–1222 (2003).
Cemerski, S. et al. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity 29, 414–422 (2008).
Acknowledgements
We thank S. Valitutti and Y. Wong for critical reading of the manuscript; A. Ting (Massachusetts Institute of Technology) for constructs for the expression of monovalent streptavidin; A. Shaw and M. Kelly (Washington University St. Louis) for AND mouse spleen cells; and Y.-H. Chien, J. Campbell, F. Wang, J. Zhou, P. Nelida, N. Sigal and A. Girvin for discussions and/or experimental assistance. Supported by the Cancer Research Institute (J.X.), the Howard Hughes Medical Institute (M.M.D.) and the US National Institutes of Health (R01 AI022511 to M.M.D.).
Author information
Authors and Affiliations
Contributions
J.X. and M.M.D. conceived of the project; J.X. developed the photocrosslinkable pMHC reagent and the acid-mediated peptide-exchange method; J.B.H. optimized the lipid bilayer system and the total internal reflection fluorescence microscope system and contributed ideas; J.X. and J.B.H. worked together on live-cell total internal reflection fluorescence imaging; E.W.N., J.H., P.J.R.E. and Q.-J.L. contributed reagents and technical support; and J.X. and M.M.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1627 kb)
Rights and permissions
About this article
Cite this article
Xie, J., Huppa, J., Newell, E. et al. Photocrosslinkable pMHC monomers stain T cells specifically and cause ligand-bound TCRs to be 'preferentially' transported to the cSMAC. Nat Immunol 13, 674–680 (2012). https://doi.org/10.1038/ni.2344
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2344
This article is cited by
-
Covalent TCR-peptide-MHC interactions induce T cell activation and redirect T cell fate in the thymus
Nature Communications (2022)
-
Monomeric TCRs drive T cell antigen recognition
Nature Immunology (2018)
-
An intermolecular FRET sensor detects the dynamics of T cell receptor clustering
Nature Communications (2017)
-
Monovalent Strep-Tactin for strong and site-specific tethering in nanospectroscopy
Nature Nanotechnology (2016)
-
CD4+ and CD8+ TCRβ repertoires possess different potentials to generate extraordinarily high-avidity T cells
Scientific Reports (2016)