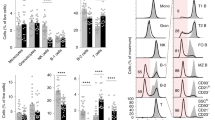
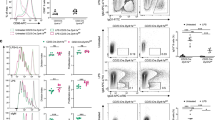
Abstract
The adaptors DOCK8 and MyD88 have been linked to serological memory. Here we report that DOCK8-deficient patients had impaired antibody responses and considerably fewer CD27+ memory B cells. B cell proliferation and immunoglobulin production driven by Toll-like receptor 9 (TLR9) were considerably lower in DOCK8-deficient B cells, but those driven by the costimulatory molecule CD40 were not. In contrast, TLR9-driven expression of AICDA (which encodes the cytidine deaminase AID), the immunoglobulin receptor CD23 and the costimulatory molecule CD86 and activation of the transcription factor NF-κB, the kinase p38 and the GTPase Rac1 were intact. DOCK8 associated constitutively with MyD88 and the tyrosine kinase Pyk2 in normal B cells. After ligation of TLR9, DOCK8 became tyrosine-phosphorylated by Pyk2, bound the Src-family kinase Lyn and linked TLR9 to a Src–kinase Syk–transcription factor STAT3 cascade essential for TLR9-driven B cell proliferation and differentiation. Thus, DOCK8 functions as an adaptor in a TLR9-MyD88 signaling pathway in B cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
24 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41590-022-01180-8
References
Yoshida, T. et al. Memory B and memory plasma cells. Immunol. Rev. 237, 117–139 (2010).
Huggins, J. et al. CpG DNA activation and plasma-cell differentiation of CD27− naive human B cells. Blood 109, 1611–1619 (2007).
Jiang, W. et al. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur. J. Immunol. 37, 2205–2213 (2007).
Giordani, L. et al. IFN-alpha amplifies human naive B cell TLR-9-mediated activation and Ig production. J. Leukoc. Biol. 86, 261–271 (2009).
Carpenter, E.L., Mick, R., Ruter, J. & Vonderheide, R.H. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J. Transl. Med. 7, 93 (2009).
Katsenelson, N. et al. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 37, 1785–1795 (2007).
Weeratna, R.D., Makinen, S.R., McCluskie, M.J. & Davis, H.L. TLR agonists as vaccine adjuvants: comparison of CpG ODN and resiquimod (R-848). Vaccine 23, 5263–5270 (2005).
Zhang, X.Q. et al. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J. Immunother. 30, 469–478 (2007).
Cunningham-Rundles, C. & Bodian, C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin. Immunol. 92, 34–48 (1999).
Latz, E. et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Immunol. 8, 772–779 (2007).
Pasare, C. & Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 438, 364–368 (2005).
Guay, H.M., Andreyeva, T.A., Garcea, R.L., Welsh, R.M. & Szomolanyi-Tsuda, E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J. Immunol. 178, 5124–5131 (2007).
Hou, B. et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34, 375–384 (2011).
Meyer-Bahlburg, A., Khim, S. & Rawlings, D.J. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J. Exp. Med. 204, 3095–3101 (2007).
Gavin, A.L. et al. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314, 1936–1938 (2006).
Park, S.M. et al. MyD88 signaling is not essential for induction of antigen-specific B cell responses but is indispensable for protection against Streptococcus pneumoniae infection following oral vaccination with attenuated Salmonella expressing PspA antigen. J. Immunol. 181, 6447–6455 (2008).
Seibert, S.A., Mex, P., Kohler, A., Kaufmann, S.H. & Mittrucker, H.W. TLR2-, TLR4- and Myd88-independent acquired humoral and cellular immunity against Salmonella enterica serovar Typhimurium. Immunol. Lett. 127, 126–134 (2010).
Sin, J.I. MyD88 signal is required for more efficient induction of Ag-specific adaptive immune responses and antitumor resistance in a human papillomavirus E7 DNA vaccine model. Vaccine 29, 4125–4131 (2011).
Browne, E.P. & Littman, D.R. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog. 5, e1000298 (2009).
Meller, N., Merlot, S. & Guda, C. CZH proteins: a new family of Rho-GEFs. J. Cell Sci. 118, 4937–4946 (2005).
Côte, J.F. & Vuori, K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 17, 383–393 (2007).
Randall, K.L. et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat. Immunol. 10, 1283–1291 (2009).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Engelhardt, K.R. et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J. Allergy Clin. Immunol. 124, 1289–1302 (2009).
Bonilla, F.A. et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann. Allergy Asthma Immunol. 94, S1–S63 (2005).
Broder, K. et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 55, 1–34 (2006).
Hartmann, G. et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164, 1617–1624 (2000).
Cunningham-Rundles, C. et al. TLR9 activation is defective in common variable immune deficiency. J. Immunol. 176, 1978–1987 (2006).
Hartmann, G. & Krieg, A.M. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164, 944–953 (2000).
Dedeoglu, F., Horwitz, B., Chaudhuri, J., Alt, F.W. & Geha, R.S. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFκB. Int. Immunol. 16, 395–404 (2004).
Arrighi, J.F., Rebsamen, M., Rousset, F., Kindler, V. & Hauser, C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 166, 3837–3845 (2001).
Lim, W. et al. Distinct role of p38 and c-Jun N-terminal kinases in IL-10-dependent and IL-10-independent regulation of the costimulatory molecule B7.2 in lipopolysaccharide-stimulated human monocytic cells. J. Immunol. 168, 1759–1769 (2002).
Kawai, T. et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5, 1061–1068 (2004).
Gotoh, K. et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J. Exp. Med. 207, 721–730 (2010).
Avery, D.T. et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207, 155–171 (2010).
Diehl, S.A. et al. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J. Immunol. 180, 4805–4815 (2008).
Barton, G.M. & Kagan, J.C. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat. Rev. Immunol. 9, 535–542 (2009).
Matsuda, T. & Hirano, T. Association of p72 tyrosine kinase with Stat factors and its activation by interleukin-3, interleukin-6, and granulocyte colony-stimulating factor. Blood 83, 3457–3461 (1994).
Uckun, F.M., Qazi, S., Ma, H., Tuel-Ahlgren, L. & Ozer, Z. STAT3 is a substrate of SYK tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress. Proc. Natl. Acad. Sci. USA 107, 2902–2907 (2010).
Avraham, H., Park, S.Y., Schinkmann, K. & Avraham, S. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 12, 123–133 (2000).
Xi, C.X., Xiong, F., Zhou, Z., Mei, L. & Xiong, W.C. PYK2 interacts with MyD88 and regulates MyD88-mediated NF-kappaB activation in macrophages. J. Leukoc. Biol. 87, 415–423 (2010).
Bonnette, P.C. et al. Phosphoproteomic characterization of PYK2 signaling pathways involved in osteogenesis. J. Proteomics 73, 1306–1320 (2010).
Gauld, S.B. & Cambier, J.C. Src-family kinases in B-cell development and signaling. Oncogene 23, 8001–8006 (2004).
Haynes, M.P. et al. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J. Biol. Chem. 278, 2118–2123 (2003).
Boudot, C. et al. Involvement of the Src kinase Lyn in phospholipase C-γ2 phosphorylation and phosphatidylinositol 3-kinase activation in Epo signalling. Biochem. Biophys. Res. Commun. 300, 437–442 (2003).
Mócsai, A., Ruland, J. & Tybulewicz, V.L. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 (2010).
Sanjuan, M.A. et al. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol. 172, 1057–1068 (2006).
Turkson, J. et al. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell Biol. 18, 2545–2552 (1998).
Shi, C.S. & Kehrl, J.H. Pyk2 amplifies epidermal growth factor and c-Src-induced Stat3 activation. J. Biol. Chem. 279, 17224–17231 (2004).
Green, N.M. & Marshak-Rothstein, A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin. Immunol. 23, 106–112 (2011).
Jabara, H.H., Fu, S.M., Geha, R.S. & Vercelli, D. CD40 and IgE: Synergism between anti-CD40 mAb and IL-4 in the induction of IgE synthesis by highly purified human B cells. J. Exp. Med. 172, 1861–1864 (1990).
Jabara, H.H., Brodeur, S.R. & Geha, R.S. Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J. Clin. Invest. 107, 371–378 (2001).
Gallego, M.D. et al. WIP and WASP play complementary roles in T cell homing and chemotaxis to SDF-1α. Int. Immunol. 18, 221–232 (2006).
Shen, X.Z., Lukacher, A.E., Billet, S., Williams, I.R. & Bernstein, K.E. Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J. Biol. Chem. 283, 9957–9965 (2008).
Acknowledgements
We thank F. Uckun (University of Southern California, Los Angeles) for SYKINH-61; R. Treisman (Cancer Research UK) for hemagglutinin-tagged MRTF-A; J.-L. Casanova (The Rockefeller University), A. Puel and C. Picard (Hopital Necker-Enfants Malades, France) for the MyD88-deficient EBV-B cell line; K. Eurich for technical assistance; A. Rambhatla and A. Chen for help in DNA sequencing; members of the Geha laboratory for discussions; J. Kagan and H. Oettgen for comments on the manuscript; and the patients and their families for donating blood. Supported by the US Public Health Service (P01AI076210, T32AI007512 and R01AI083503 to R.S.G.; R21AI087627 to T.A.C.; and K08AI076625 to D.R.M.), the Dubai Harvard Foundation for Medical Research (R.S.G. and L.D.N.), the Swiss National Science Foundation (PASMP3-127678/1 to M.R.), the Clinical Immunology Society (E.J.) and the Manton Foundation (E.J. and L.D.N.).
Author information
Authors and Affiliations
Contributions
H.H.J., D.R.M., E.J., M.J.M., N.R., A.B., I.Ra., H.B. and M.R. did the experiments; L.S., S.B., R.W., G.D., M.D., W.A.-H., I.B., S.B., N.K., H.D.O., A.P., M.K., G.L. and I.Re. provided patient blood samples; K.A.F. and D.G. provided mice; J.M. and L.D.N. provided advice; S.K., R.C. and T.A.C. sequenced DNA; and H.H.J. and R.S.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Tables 1–3 (PDF 18208 kb)
Rights and permissions
About this article
Cite this article
Jabara, H., McDonald, D., Janssen, E. et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol 13, 612–620 (2012). https://doi.org/10.1038/ni.2305
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2305