Abstract
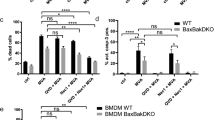
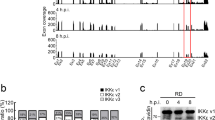
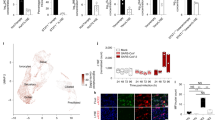
The innate immune system limits viral replication via type I interferon and also induces the presentation of viral antigens to cells of the adaptive immune response. Using infection of mice with vesicular stomatitis virus, we analyzed how the innate immune system inhibits viral propagation but still allows the presentation of antigen to cells of the adaptive immune response. We found that expression of the gene encoding the inhibitory protein Usp18 in metallophilic macrophages led to lower type I interferon responsiveness, thereby allowing locally restricted replication of virus. This was essential for the induction of adaptive antiviral immune responses and, therefore, for preventing the fatal outcome of infection. In conclusion, we found that enforced viral replication in marginal zone macrophages was an immunological mechanism that ensured the production of sufficient antigen for effective activation of the adaptive immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Müller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Sadler, A.J. & Williams, B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8, 559–568 (2008).
Aichele, P. et al. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 171, 1148–1155 (2003).
Cervantes-Barragán, L. et al. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J. Immunol. 182, 1099–1106 (2009).
Lang, P.A. et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology 52, 25–32 (2010).
Seiler, P. et al. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 27, 2626–2633 (1997).
Steiniger, B. & Barth, P. Microanatomy and function of the spleen. Adv. Anat. Embryol. Cell Biol. 151, III–IX, 1–101 (2000).
Wardle, E.N. Kupffer cells and their function. Liver 7, 63–75 (1987).
Kraal, G., Ter Hart, H., Meelhuizen, C., Venneker, G. & Claassen, E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur. J. Immunol. 19, 675–680 (1989).
Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Der, S.D., Zhou, A., Williams, B.R. & Silverman, R.H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95, 15623–15628 (1998).
Ritchie, K.J. et al. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 10, 1374–1378 (2004).
Malakhova, O.A. et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 25, 2358–2367 (2006).
Junt, T., Scandella, E. & Ludewig, B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat. Rev. Immunol. 8, 764–775 (2008).
Oetke, C., Kraal, G. & Crocker, P.R. The antigen recognized by MOMA-I is sialoadhesin. Immunol. Lett. 106, 96–98 (2006).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Gretz, J.E., Anderson, A.O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Aichele, P., Brduscha-Riem, K., Zinkernagel, R.M., Hengartner, H. & Pircher, H. T cell priming versus T cell tolerance induced by synthetic peptides. J. Exp. Med. 182, 261–266 (1995).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8, 89–95 (1998).
Lanzavecchia, A. & Sallusto, F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat. Immunol. 2, 487–492 (2001).
Zinkernagel, R.M. Localization dose and time of antigens determine immune reactivity. Semin. Immunol. 12, 163–171, discussion 257–344 (2000).
Zinkernagel, R.M. et al. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156, 199–209 (1997).
Irvine, D.J., Purbhoo, M.A., Krogsgaard, M. & Davis, M.M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Henrickson, S.E. et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat. Immunol. 9, 282–291 (2008).
Holler, P.D. & Kranz, D.M. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18, 255–264 (2003).
Bachmann, M.F., Bast, C., Hengartner, H. & Zinkernagel, R.M. Immunogenicity of a viral model vaccine after different inactivation procedures. Med. Microbiol. Immunol. (Berl.) 183, 95–104 (1994).
Miyawaki, S. et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24, 429–434 (1994).
Miyake, Y. et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Invest. 117, 2268–2278 (2007).
Bachmann, M.F., Kundig, T.M., Kalberer, C.P., Hengartner, H. & Zinkernagel, R.M. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J. Virol. 67, 3917–3922 (1993).
Freer, G. et al. Role of T helper cell precursor frequency on vesicular stomatitis virus neutralizing antibody responses in a T cell receptor β chain transgenic mouse. Eur. J. Immunol. 25, 1410–1416 (1995).
Nonacs, R., Humborg, C., Tam, J.P. & Steinman, R.M. Mechanisms of mouse spleen dendritic cell function in the generation of influenza-specific, cytolytic T lymphocytes. J. Exp. Med. 176, 519–529 (1992).
Bründler, M.A. et al. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 26, 2257–2262 (1996).
Plakhov, I.V., Arlund, E.E., Aoki, C. & Reiss, C.S. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology 209, 257–262 (1995).
Junt, T. et al. Expression of lymphotoxin β governs immunity at two distinct levels. Eur. J. Immunol. 36, 2061–2075 (2006).
Ware, C.F. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol. Rev. 223, 186–201 (2008).
Seifert, U. et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142, 613–624 (2010).
Le Bon, A. et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4, 1009–1015 (2003).
Longhi, M.P. et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206, 1589–1602 (2009).
Kolumam, G.A., Thomas, S., Thompson, L.J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Fink, K. et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur. J. Immunol. 36, 2094–2105 (2006).
Iannacone, M. et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 (2010).
Yoshimura, A. Negative regulation of cytokine signaling. Clin. Rev. Allergy Immunol. 28, 205–220 (2005).
Battegay, M. et al. Antiviral immune responses of mice lacking MHC class II or its associated invariant chain. Cell. Immunol. 167, 115–121 (1996).
Ogra, P.L., Karzon, D.T., Righthand, F. & MacGillivray, M. Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N. Engl. J. Med. 279, 893–900 (1968).
Ogra, P.L., Kerr-Grant, D., Umana, G., Dzierba, J. & Weintraub, D. Antibody response in serum and nasopharynx after naturally acquired and vaccine-induced infection with rubella virus. N. Engl. J. Med. 285, 1333–1339 (1971).
Ida-Hosonuma, M. et al. The α/β interferon response controls tissue tropism and pathogenicity of poliovirus. J. Virol. 79, 4460–4469 (2005).
Ohashi, P.S. et al. Induction of diabetes is influenced by the infectious virus and local expression of MHC class I and tumor necrosis factor-α. J. Immunol. 150, 5185–5194 (1993).
Lang, K.S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 11, 138–145 (2005).
Millar, D.G. et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat. Med. 9, 1469–1476 (2003).
Klingel, K. et al. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of viral replication, tissue damage, and inflammation. Proc. Natl. Acad. Sci. USA 89, 314–318 (1992).
Acknowledgements
We thank D. Kolakofsky (University of Geneva) for VSV; and K. Schättel for technical support. Supported by the Alexander von Humboldt Foundation (SKA-2008 to K.S.L. and SKA-2010 to P.A.L.), Collaborative Research Center SFB575, Experimental Hepatology (coordinator, D.H.; Deutsche Forschungsgemeinschaft grant LA1419/3-1 to K.S.L. and SFB-TR19 to K.K. and R.K.; FOR729 to K.P.; SFB/Transregio 60 (coordinator, M. Roggendorf), the MOI Manchot Graduate School (Jürgen Manchot Foundation), the Swiss National Science Foundation (PASMP3-127678/1 to M.R.) and the US National Institutes of Health (R01 HL091549 for Usp18-related work in the D.-E.Z. laboratory).
Author information
Authors and Affiliations
Contributions
N.H. planned and did most experiments; N.S. planned and did several experiments; G.C. and S.E.B. did laser-capture dissection; U.R.S. contributed to Ltbr−/− mouse experiments; D.-E.Z. contributed to experiments on Usp18; M.T. and C.B. contributed to transfection experiments; K.K., M.S. and R.K. did and interpreted in situ hybridization; N.G. did in vitro experiments; N.v.R. contributed to macrophage depletion experiments; M.G. did in vitro stimulation of DCs; M.L., H.H., K.P., M.T., D.H. and M.R. discussed and interpreted data and helped to write the manuscript; and P.A.L. and K.S.L. initiated and designed the study and wrote most of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 969 kb)
Rights and permissions
About this article
Cite this article
Honke, N., Shaabani, N., Cadeddu, G. et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 13, 51–57 (2012). https://doi.org/10.1038/ni.2169
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2169
This article is cited by
-
USP18 enhances dengue virus replication by regulating mitochondrial DNA release
Scientific Reports (2023)
-
Differential kinetics of splenic CD169+ macrophage death is one underlying cause of virus infection fate regulation
Cell Death & Disease (2023)
-
Enzyme-derived deer velvet extract activate the immune response in cyclophosphamide-induced immunosuppressive mice
Food Science and Biotechnology (2023)
-
A detailed analysis of F-MuLV- and SFFV-infected cells in Friend virus-infected mice reveals the contribution of both F-MuLV- and SFFV-infected cells to the interleukin-10 host response
Retrovirology (2022)
-
Repeated dosing improves oncolytic rhabdovirus therapy in mice via interactions with intravascular monocytes
Communications Biology (2022)