Abstract
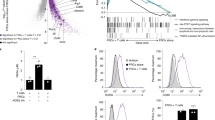
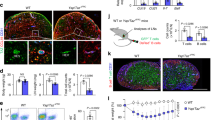
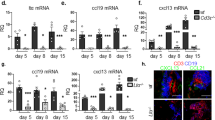
Fibroblastic reticular cells (FRCs) and lymphatic endothelial cells (LECs) are nonhematopoietic stromal cells of lymphoid organs. They influence the migration and homeostasis of naive T cells; however, their influence on activated T cells remains undescribed. Here we report that FRCs and LECs inhibited T cell proliferation through a tightly regulated mechanism dependent on nitric oxide synthase 2 (NOS2). Expression of NOS2 and production of nitric oxide paralleled the activation of T cells and required a tripartite synergism of interferon-γ, tumor necrosis factor and direct contact with activated T cells. Notably, in vivo expression of NOS2 by FRCs and LECs regulated the size of the activated T cell pool. Our study elucidates an as-yet-unrecognized role for the lymph node stromal niche in controlling T cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mueller, S.N. & Ahmed, R. Lymphoid stroma in the initiation and control of immune responses. Immunol. Rev. 224, 284–294 (2008).
Katakai, T., Hara, T., Sugai, M., Gonda, H. & Shimizu, A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200, 783–795 (2004).
Gretz, J.E., Anderson, A.O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997).
Bajénoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Mueller, S.N. & Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9, 618–629 (2009).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007).
Thomas, S., Kolumam, G.A. & Murali-Krishna, K. Antigen presentation by nonhemopoietic cells amplifies clonal expansion of effector CD8 T cells in a pathogen-specific manner. J. Immunol. 178, 5802–5811 (2007).
Fletcher, A.L. et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J. Exp. Med. 207, 689–697 (2010).
Lee, J.W. et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 8, 181–190 (2007).
Magnusson, F.C. et al. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology 134, 1028–1037 (2008).
Nichols, L.A. et al. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J. Immunol. 179, 993–1003 (2007).
Grigorova, I.L. et al. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 10, 58–65 (2009).
Pham, T.H. et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17–27 (2010).
Cohen, J.N. et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 207, 681–688 (2010).
Angeli, V. et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 (2006).
Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2, 907–916 (2001).
Krumenacker, J.S., Hanafy, K.A. & Murad, F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res. Bull. 62, 505–515 (2004).
Barcellos, L.F. et al. Genetic variation in nitric oxide synthase 2A (NOS2A) and risk for multiple sclerosis. Genes Immun. 9, 493–500 (2008).
Kato, H. et al. Effect of NOS2 gene deficiency on the development of autoantibody mediated arthritis and subsequent articular cartilage degeneration. J. Rheumatol. 30, 247–255 (2003).
Kolios, G., Valatas, V. & Ward, S.G. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology 113, 427–437 (2004).
Stuart, P.E. et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet. 42, 1000–1004 (2010).
Niedbala, W., Cai, B. & Liew, F.Y. Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 65 (suppl. 3), iii37–iii40 (2006).
Niedbala, W. et al. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc. Natl. Acad. Sci. USA 104, 15478–15483 (2007).
Nathan, C. & Xie, Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 269, 13725–13728 (1994).
Duthoit, C.T., Mekala, D.J., Alli, R.S. & Geiger, T.L. Uncoupling of IL-2 signaling from cell cycle progression in naive CD4+ T cells by regulatory CD4+CD25+ T lymphocytes. J. Immunol. 174, 155–163 (2005).
Hu, X. & Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009).
Gabrilovich, D.I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Mueller, S.N. et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc. Natl. Acad. Sci. USA 104, 15430–15435 (2007).
Reynoso, E.D. et al. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J. Immunol. 182, 2102–2112 (2009).
Grohmann, U. & Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 236, 243–264 (2010).
Vig, M. et al. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J. Clin. Invest. 113, 1734–1742 (2004).
Vezys, V., Olson, S. & Lefrancois, L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity 12, 505–514 (2000).
Buettner, M., Pabst, R. & Bode, U. Lymph node stromal cells strongly influence immune response suppression. Eur. J. Immunol. 41, 624–633 (2011).
Jones, S., Horwood, N., Cope, A. & Dazzi, F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J. Immunol. 179, 2824–2831 (2007).
Krampera, M. et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24, 386–398 (2006).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008).
Aggarwal, S. & Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 (2005).
Podgrabinska, S. et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol. 183, 1767–1779 (2009).
Francisco, L.M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
English, K., Barry, F.P., Field-Corbett, C.P. & Mahon, B.P. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 110, 91–100 (2007).
Kamijo, R. et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263, 1612–1615 (1994).
Saura, M., Zaragoza, C., Bao, C., McMillan, A. & Lowenstein, C.J. Interaction of interferon regulatory factor-1 and nuclear factor κB during activation of inducible nitric oxide synthase transcription. J. Mol. Biol. 289, 459–471 (1999).
Farlik, M. et al. Nonconventional initiation complex assembly by STAT and NF-κB transcription factors regulates nitric oxide synthase expression. Immunity 33, 25–34 (2010).
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002).
Wei, X.Q. et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375, 408–411 (1995).
Huang, S. et al. Immune response in mice that lack the interferon-γ receptor. Science 259, 1742–1745 (1993).
Badovinac, V.P., Tvinnereim, A.R. & Harty, J.T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290, 1354–1358 (2000).
Feuerer, M., Eulenburg, K., Loddenkemper, C., Hamann, A. & Huehn, J. Self-limitation of Th1-mediated inflammation by IFN-γ. J. Immunol. 176, 2857–2863 (2006).
Kahn, D.A., Archer, D.C., Gold, D.P. & Kelly, C.J. Adjuvant immunotherapy is dependent on inducible nitric oxide synthase. J. Exp. Med. 193, 1261–1268 (2001).
Acknowledgements
We thank M. Curry for technical assistance at the Dana-Farber Cancer Institute Flow Cytometry Core Facility; A. Sharpe (Harvard Medical School) for PD-L1-deficient mice; L. Lefrancois (University of Connecticut) for iFABP-tOVA mice; L.-H. Ang, Y. Zheng and S.J. Hagen for technical assistance at the Imaging Microscopy Core of Beth Israel Deaconess Medical Center; and J. Astarita and A. Bellemare-Pelletier for critically reading the manuscript. Supported by the US National Institutes of Health (R01 DK074500 and P01 AI045757 to S.J.T.) and the Dana-Farber Cancer Institute (V.L.-K).
Author information
Authors and Affiliations
Contributions
V.L.-K. designed and did most of the experiments, analyzed and interpreted data and wrote the manuscript; D.M. did individual experiments and discussed and interpreted results; A.L.F. edited the manuscript; A.L.F., S.E.A., K.G.E., P.T. and A.C. discussed and interpreted results and provided technical help for the experiments; and S.J.T. directed the study, analyzed and interpreted results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Methods (PDF 766 kb)
Rights and permissions
About this article
Cite this article
Lukacs-Kornek, V., Malhotra, D., Fletcher, A. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 12, 1096–1104 (2011). https://doi.org/10.1038/ni.2112
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2112
This article is cited by
-
Biomechanical control of lymphatic vessel physiology and functions
Cellular & Molecular Immunology (2023)
-
New fibroblast network connections support lymphocytic cellular service
Nature Immunology (2023)
-
Lymphoid stromal cells proGrem dendritic cell homeostasis
Nature Immunology (2021)
-
Quantitation of lymphatic transport mechanism and barrier influences on lymph node-resident leukocyte access to lymph-borne macromolecules and drug delivery systems
Drug Delivery and Translational Research (2021)
-
Lymph node stromal cells: cartographers of the immune system
Nature Immunology (2020)