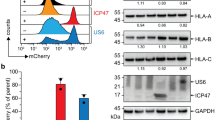
Abstract
The surface presentation of peptides by major histocompatibility complex (MHC) class I molecules is critical to CD8+ T cell–mediated adaptive immune responses. Aminopeptidases have been linked to the editing of peptides for MHC class I loading, but carboxy-terminal editing is thought to be due to proteasome cleavage. By analysis of wild-type mice and mice genetically deficient in or overexpressing the dipeptidase angiotensin-converting enzyme (ACE), we have now identified ACE as having a physiological role in the processing of peptides for MHC class I. ACE edited the carboxyl terminus of proteasome-produced MHC class I peptides. The lack of ACE exposed new antigens but also abrogated some self antigens. ACE had substantial effects on the surface expression of MHC class I in a haplotype-dependent manner. We propose a revised model of peptide processing for MHC class I by introducing carboxypeptidase activity into the process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jensen, P.E. Recent advances in antigen processing and presentation. Nat. Immunol. 8, 1041–1048 (2007).
Wearsch, P.A. & Cresswell, P. The quality control of MHC class I peptide loading. Curr. Opin. Cell Biol. 20, 624–631 (2008).
Purcell, A.W. & Elliott, T. Molecular machinations of the MHC-I peptide loading complex. Curr. Opin. Immunol. 20, 75–81 (2008).
Rock, K.L., York, I.A. & Goldberg, A.L. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat. Immunol. 5, 670–677 (2004).
Rammensee, H.G., Friede, T. & Stevanoviíc, S. MHC ligands and peptide motifs: first listing. Immunogenetics 41, 178–228 (1995).
Saveanu, L., Carroll, O., Hassainya, Y. & van Endert, P. Complexity, contradictions, and conundrums: studying post-proteasomal proteolysis in HLA class I antigen presentation. Immunol. Rev. 207, 42–59 (2005).
Serwold, T., Gonzalez, F., Kim, J., Jacob, R. & Shastri, N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419, 480–483 (2002).
Saric, T. et al. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3, 1169–1176 (2002).
Mo, X.Y., Cascio, P., Lemerise, K., Goldberg, A.L. & Rock, K. Distinct proteolytic processes generate the C and N termini of MHC class I-binding peptides. J. Immunol. 163, 5851–5859 (1999).
Rammensee, H.G. Peptides made to order. Immunity 25, 693–695 (2006).
Kessler, J.H. et al. Antigen processing by nardilysin and thimet oligopeptidase generates cytotoxic T cell epitopes. Nat. Immunol. 12, 45–53 (2011).
Hooper, N.M. Angiotensin converting enzyme: implications from molecular biology for its physiological functions. Int. J. Biochem. 23, 641–647 (1991).
Corvol, P., Williams, T.A. & Soubrier, F. Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 248, 283–305 (1995).
Shen, X.Z., Lukacher, A.E., Billet, S., Williams, I.R. & Bernstein, K.E. Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J. Biol. Chem. 283, 9957–9965 (2008).
Harmer, D., Gilbert, M., Borman, R. & Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 532, 107–110 (2002).
Danilov, S.M. et al. Angiotensin-converting enzyme (CD143) is abundantly expressed by dendritic cells and discriminates human monocyte-derived dendritic cells from acute myeloid leukemia-derived dendritic cells. Exp. Hematol. 31, 1301–1309 (2003).
Saijonmaa, O., Nyman, T. & Fyhrquist, F. Atorvastatin inhibits angiotensin-converting enzyme induction in differentiating human macrophages. Am. J. Physiol. Heart Circ. Physiol. 292, H1917–H1921 (2007).
Eisenlohr, L.C., Bacik, I., Bennink, J.R., Bernstein, K. & Yewdell, J.W. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell 71, 963–972 (1992).
Kozlowski, S. et al. Serum angiotensin-1 converting enzyme activity processes a human immunodeficiency virus 1 gp160 peptide for presentation by major histocompatibility complex class I molecules. J. Exp. Med. 175, 1417–1422 (1992).
Strehl, B. et al. Interferon-γ, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol. Rev. 207, 19–30 (2005).
Esther, C.R. et al. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab. Invest. 74, 953–965 (1996).
Shen, X.Z. et al. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am. J. Pathol. 170, 2122–2134 (2007).
Hammer, G.E., Gonzalez, F., James, E., Nolla, H. & Shastri, N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat. Immunol. 8, 101–108 (2007).
Simpson, E., Scott, D. & Chandler, P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu. Rev. Immunol. 15, 39–61 (1997).
Greenfield, A. et al. An H-YDb epitope is encoded by a novel mouse Y chromosome gene. Nat. Genet. 14, 474–478 (1996).
Kemball, C.C. et al. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J. Immunol. 174, 7950–7960 (2005).
Andrews, N.P., Pack, C.D. & Lukacher, A.E. Generation of antiviral major histocompatibility complex class I-restricted T cells in the absence of CD8 coreceptors. J. Virol. 82, 4697–4705 (2008).
Cushman, D.W., Cheung, H.S., Sabo, E.F. & Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 16, 5484–5491 (1977).
Kanaseki, T., Blanchard, N., Hammer, G.E., Gonzalez, F. & Shastri, N. ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the endoplasmic reticulum. Immunity 25, 795–806 (2006).
Fuchs, S. et al. Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J. Biol. Chem. 279, 15946–15953 (2004).
Craiu, A., Akopian, T., Goldberg, A. & Rock, K.L. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc. Natl. Acad. Sci. USA 94, 10850–10855 (1997).
Stoltze, L. et al. Generation of the vesicular stomatitis virus nucleoprotein cytotoxic T lymphocyte epitope requires proteasome-dependent and -independent proteolytic activities. Eur. J. Immunol. 28, 4029–4036 (1998).
Kunisawa, J. & Shastri, N. The group II chaperonin TRiC protects proteolytic intermediates from degradation in the MHC class I antigen processing pathway. Mol. Cell 12, 565–576 (2003).
Skidgel, R.A. & Erdös, E.G. Angiotensin I converting enzyme. Adv. Exp. Med. Biol. 247A, 25–28 (1989).
Hemming, M.L. & Selkoe, D.J. Amyloid β-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J. Biol. Chem. 280, 37644–37650 (2005).
Chang, S.C., Momburg, F., Bhutani, N. & Goldberg, A.L. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl. Acad. Sci. USA 102, 17107–17112 (2005).
Androlewicz, M.J. & Cresswell, P. How selective is the transporter associated with antigen processing? Immunity 5, 1–5 (1996).
Koopmann, J.O., Post, M., Neefjes, J.J., Hämmerling, G.J. & Momburg, F. Translocation of long peptides by transporters associated with antigen processing (TAP). Eur. J. Immunol. 26, 1720–1728 (1996).
Schumacher, T.N. et al. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J. Exp. Med. 179, 533–540 (1994).
Momburg, F. et al. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature 367, 648–651 (1994).
Lauterbach, H., Gruber, A., Ried, C., Cheminay, C. & Brocker, T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J. Immunol. 176, 4600–4607 (2006).
Cole, J. et al. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J. Clin. Invest. 106, 1391–1398 (2000).
Acknowledgements
We thank S. Fuchs and E. Bernstein for vector construction; D. Roopenian and G. Christianson (Jackson Laboratories) for the minor histocompatibility CTL clones and assistance with cultures; G. Khitrov for assistance in liquid chromatography–mass spectrometry; G.E. Hammer for technical assistance with immunization; Q. Xu for assistance with RT-PCR; S. McLachlan (Cedars-Sinai Medical Center) for A20 cells; C. Ried (University of Munich) for Cd11c promoter DNA; R. Germain (US National Institutes of Health) for antibody 25D-1.16; R. Ahmed (Emory University) for L. monocytogenes strain EGD; and B. Taylor for administrative support. Supported by the US National Institutes of Health (R01 DK 039777 to K.E.B. and R01 CA 71971 to A.E.L.).
Author information
Authors and Affiliations
Contributions
X.Z.S., study conception and experimental design; X.Z.S. and S.B., experimental input, with assistance from C.L. (RT-PCR), D.O.-D. (L. monocytogenes infection) and X.C. (minigene construction); A.E.L., intellectual advice and reagents for polyomavirus experiments; K.E.B., intellectual advice and project coordination; X.Z.S. and K.E.B., manuscript authorship.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Table 1 (PDF 752 kb)
Rights and permissions
About this article
Cite this article
Shen, X., Billet, S., Lin, C. et al. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol 12, 1078–1085 (2011). https://doi.org/10.1038/ni.2107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2107
This article is cited by
-
The Relationship Between Serum Angiotensin Converting Enzyme Level and the Decision to Escalate Treatment of Sarcoidosis
Lung (2023)
-
Role of angiotensin-converting enzyme in myeloid cell immune responses
Cellular & Molecular Biology Letters (2020)
-
Overexpression of ACE in Myeloid Cells Increases Immune Effectiveness and Leads to a New Way of Considering Inflammation in Acute and Chronic Diseases
Current Hypertension Reports (2020)
-
Angiotensin-converting enzyme in innate and adaptive immunity
Nature Reviews Nephrology (2018)
-
Angiotensin-converting enzyme affects the presentation of MHC class II antigens
Laboratory Investigation (2017)