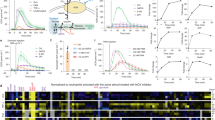
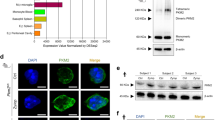
Abstract
Inositol phosphates are widely produced throughout animal and plant tissues. Diphosphoinositol pentakisphosphate (InsP7) contains an energetic pyrophosphate bond. Here we demonstrate that disruption of inositol hexakisphosphate kinase 1 (InsP6K1), one of the three mammalian inositol hexakisphosphate kinases (InsP6Ks) that convert inositol hexakisphosphate (InsP6) to InsP7, conferred enhanced phosphatidylinositol-(3,4,5)-trisphosphate (PtdIns(3,4,5)P3)-mediated membrane translocation of the pleckstrin homology domain of the kinase Akt and thus augmented downstream PtdIns(3,4,5)P3 signaling in mouse neutrophils. Consequently, these neutrophils had greater phagocytic and bactericidal ability and amplified NADPH oxidase–mediated production of superoxide. These phenotypes were replicated in human primary neutrophils with pharmacologically inhibited InsP6Ks. In contrast, an increase in intracellular InsP7 blocked chemoattractant-elicited translocation of the pleckstrin homology domain to the membrane and substantially suppressed PtdIns(3,4,5)P3-mediated cellular events in neutrophils. Our findings establish a role for InsP7 in signal transduction and provide a mechanism for modulating PtdIns(3,4,5)P3 signaling in neutrophils.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Shears, S.B. Diphosphoinositol polyphosphates: metabolic messengers? Mol. Pharmacol. 76, 236–252 (2009).
Stephens, L. et al. The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s). J. Biol. Chem. 268, 4009–4015 (1993).
Shears, S.B. How versatile are inositol phosphate kinases? Biochem. J. 377, 265–280 (2004).
Burton, A., Hu, X. & Saiardi, A. Are inositol pyrophosphates signalling molecules? J. Cell. Physiol. 220, 8–15 (2009).
Menniti, F.S., Miller, R.N., Putney, J.W. Jr. & Shears, S.B. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J. Biol. Chem. 268, 3850–3856 (1993).
Glennon, M.C. & Shears, S.B. Turnover of inositol pentakisphosphates, inositol hexakisphosphate and diphosphoinositol polyphosphates in primary cultured hepatocytes. Biochem. J. 293, 583–590 (1993).
Safrany, S.T. & Shears, S.B. Turnover of bis-diphosphoinositol tetrakisphosphate in a smooth muscle cell line is regulated by β2-adrenergic receptors through a cAMP-mediated, A-kinase-independent mechanism. EMBO J. 17, 1710–1716 (1998).
Illies, C. et al. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science 318, 1299–1302 (2007).
Luo, H.R. et al. GRAB: a physiologic guanine nucleotide exchange factor for Rab3A, which interacts with inositol hexakisphosphate kinase. Neuron 31, 439–451 (2001).
Morrison, B.H., Bauer, J.A., Kalvakolanu, D.V. & Lindner, D.J. Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-β in ovarian carcinoma cells. J. Biol. Chem. 276, 24965–24970 (2001).
Morrison, B.H. et al. Inositol hexakisphosphate kinase 2 sensitizes ovarian carcinoma cells to multiple cancer therapeutics. Oncogene 21, 1882–1889 (2002).
Nagata, E. et al. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J. Biol. Chem. 280, 1634–1640 (2005).
Chakraborty, A. et al. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc. Natl. Acad. Sci. USA 105, 1134–1139 (2008).
Bhandari, R., Juluri, K.R., Resnick, A.C. & Snyder, S.H. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc. Natl. Acad. Sci. USA 105, 2349–2353 (2008).
Chakraborty, A. et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 897–910 (2010).
Luo, H.R. et al. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 114, 559–572 (2003).
Iijima, M., Huang, Y.E. & Devreotes, P. Temporal and spatial regulation of chemotaxis. Dev. Cell 3, 469–478 (2002).
Stephens, L., Ellson, C. & Hawkins, P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr. Opin. Cell Biol. 14, 203–213 (2002).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Lemmon, M.A. Phosphoinositide recognition domains. Traffic 4, 201–213 (2003).
Subramanian, K.K. et al. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood 109, 4028–4037 (2007).
Jia, Y. et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates PtdIns(3,4,5)P3 signaling in neutrophils. Immunity 27, 453–467 (2007).
Parent, C.A., Blacklock, B.J., Froehlich, W.M., Murphy, D.B. & Devreotes, P.N. G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91 (1998).
Thomas, C.C., Deak, M., Alessi, D.R. & van Aalten, D.M. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr. Biol. 12, 1256–1262 (2002).
Dinauer, M.C. Chronic granulomatous disease and other disorders of phagocyte function. Hematology (Am. Soc. Hematol. Educ. Program) 89–95 (2005).
Matute, J.D., Arias, A.A., Dinauer, M.C. & Patino, P.J. p40phox: the last NADPH oxidase subunit. Blood Cells Mol. Dis. 35, 291–302 (2005).
Dahlgren, C., Johansson, A. & Orselius, K. Difference in hydrogen peroxide release between human neutrophils and neutrophil cytoplasts following calcium ionophore activation. A role of the subcellular granule in activation of the NADPH-oxidase in human neutrophils? Biochim. Biophys. Acta 1010, 41–48 (1989).
Dinauer, M.C. & Orkin, S.H. Chronic granulomatous disease. Annu. Rev. Med. 43, 117–124 (1992).
Parekh, D.B., Ziegler, W. & Parker, P.J. Multiple pathways control protein kinase C phosphorylation. EMBO J. 19, 496–503 (2000).
Baumer, A.T. et al. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for α-platelet-derived growth factor receptor-induced production of reactive oxygen species. J. Biol. Chem. 283, 7864–7876 (2008).
Chen, Q. et al. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J. Immunol. 170, 5302–5308 (2003).
Padmanabhan, U., Dollins, D.E., Fridy, P.C., York, J.D. & Downes, C.P. Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates. J. Biol. Chem. 284, 10571–10582 (2009).
Brown, G.E., Stewart, M.Q., Liu, H., Ha, V.L. & Yaffe, M.B. A novel assay system implicates PtdIns(3,4)P2, PtdIns(3)P, and PKCδ in intracellular production of reactive oxygen species by the NADPH oxidase. Mol. Cell 11, 35–47 (2003).
Li, Y. et al. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 113, 4930–4941 (2009).
Clark, R.A. & Klebanoff, S.J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J. Clin. Invest. 64, 913–920 (1979).
Segal, B.H., Kuhns, D.B., Ding, L., Gallin, J.I. & Holland, S.M. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47phox: complement, leukotrienes, and reactive oxidants in acute inflammation. J. Leukoc. Biol. 71, 410–416 (2002).
Lekstrom-Himes, J.A., Kuhns, D.B., Alvord, W.G. & Gallin, J.I. Inhibition of human neutrophil IL-8 production by hydrogen peroxide and dysregulation in chronic granulomatous disease. J. Immunol. 174, 411–417 (2005).
Heit, B. et al. PTEN functions to 'prioritize' chemotactic cues and prevent 'distraction' in migrating neutrophils. Nat. Immunol. 9, 743–752 (2008).
Liu, L., Puri, K.D., Penninger, J.M. & Kubes, P. Leukocyte PI3Kγ and PI3Kδ have temporally distinct roles for leukocyte recruitment in vivo. Blood 110, 1191–1198 (2007).
Ferguson, G.J. et al. PI(3)Kγ has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 9, 86–91 (2007).
Lee, Y.S., Mulugu, S., York, J.D. & O'Shea, E.K. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316, 109–112 (2007).
Voglmaier, S.M. et al. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem. Biophys. Res. Commun. 187, 158–163 (1992).
Norris, F.A., Ungewickell, E. & Majerus, P.W. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP-3/AP180) and inhibits clathrin cage assembly in vitro. J. Biol. Chem. 270, 214–217 (1995).
Ye, W., Ali, N., Bembenek, M.E., Shears, S.B. & Lafer, E.M. Inhibition of clathrin assembly by high affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J. Biol. Chem. 270, 1564–1568 (1995).
Saiardi, A., Bhandari, R., Resnick, A.C., Snowman, A.M. & Snyder, S.H. Phosphorylation of proteins by inositol pyrophosphates. Science 306, 2101–2105 (2004).
Bhandari, R. et al. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc. Natl. Acad. Sci. USA 104, 15305–15310 (2007).
Tan, B.L. et al. Genetic evidence for convergence of c-Kit- and alpha4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood 101, 4725–4732 (2003).
Li, Z. et al. Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 7, 399–404 (2005).
Zhu, D. et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc. Natl. Acad. Sci. USA 103, 14836–14841 (2006).
Jia, Y. et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5-trisphosphate signaling in neutrophils. Immunity 27, 453–467 (2007).
Acknowledgements
We thank L. Silberstein, J. Manis and L. Chai for discussions. Supported by the US National Institutes of Health (HL085100, AI076471, HL092020 and GM076084 to H.R.L.).
Author information
Authors and Affiliations
Contributions
A.P., Y.J., A.C., S.H.S. and H.R.L. designed the experiments; A.P., Y.J., A.C., Y.L., S.K.J., J.Z., S.G.R., F.L., S.M., J.S. and C.B. did the experiments; A.P., Y.J., A.C., S.H.S. and H.R.L. analyzed data; and A.P., Y.J., S.H.S. and H.R.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 1117 kb)
Supplementary Movie 1
Chemoattractant-elicited polarization of WT neutrophils (MOV 2006 kb)
Supplementary Movie 2
Chemoattractant-elicited polarization of InsP6K−/− neutrophils (MOV 1696 kb)
Supplementary Movie 3
Adhesion of WT (left) and InsP6K−/− (right) neutrophils under shear flow (MOV 799 kb)
Supplementary Movie 4
Detachment of adhered WT (left) and InsP6K−/− (right) neutrophils under shear flow (MOV 1119 kb)
Supplementary Movie 5
Chemotaxis of WT neutrophils (bottom) towards 1 AM of fMLP (top) in EZ-TAXIScan chamber (MOV 882 kb)
Supplementary Movie 6
Chemotaxis of InsP6K1−/− neutrophils (bottom) towards 1 AM of fMLP (top) in EZ-TAXIScan chamber (MOV 862 kb)
Rights and permissions
About this article
Cite this article
Prasad, A., Jia, Y., Chakraborty, A. et al. Inositol hexakisphosphate kinase 1 regulates neutrophil function in innate immunity by inhibiting phosphatidylinositol-(3,4,5)-trisphosphate signaling. Nat Immunol 12, 752–760 (2011). https://doi.org/10.1038/ni.2052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2052
This article is cited by
-
Functions, Mechanisms, and therapeutic applications of the inositol pyrophosphates 5PP-InsP5 and InsP8 in mammalian cells
Journal of Cardiovascular Translational Research (2023)
-
Inositol treatment inhibits medulloblastoma through suppression of epigenetic-driven metabolic adaptation
Nature Communications (2021)
-
L-selectin mechanochemistry restricts neutrophil priming in vivo
Nature Communications (2017)
-
Inositol Pyrophosphates: Energetic, Omnipresent and Versatile Signalling Molecules
Journal of the Indian Institute of Science (2017)
-
New Synthetic Methods for Phosphate Labeling
Topics in Current Chemistry (2017)