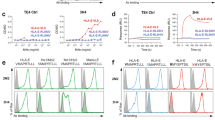
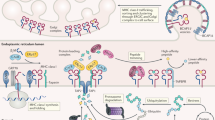
Abstract
Cytotoxic T lymphocytes (CTLs) recognize peptides presented by HLA class I molecules on the cell surface. The C terminus of these CTL epitopes is considered to be produced by the proteasome. Here we demonstrate that the cytosolic endopeptidases nardilysin and thimet oligopeptidase (TOP) complemented proteasome activity. Nardilysin and TOP were required, either together or alone, for the generation of a tumor-specific CTL epitope from PRAME, an immunodominant CTL epitope from Epstein-Barr virus protein EBNA3C, and a clinically important epitope from the melanoma protein MART-1. TOP functioned as C-terminal trimming peptidase in antigen processing, and nardilysin contributed to both the C-terminal and N-terminal generation of CTL epitopes. By broadening the antigenic peptide repertoire, nardilysin and TOP strengthen the immune defense against intracellular pathogens and cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Rock, K.L., York, I.A. & Goldberg, A.L. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat. Immunol. 5, 670–677 (2004).
Shastri, N., Cardinaud, S., Schwab, S.R., Serwold, T. & Kunisawa, J. All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol. Rev. 207, 31–41 (2005).
Yewdell, J.W. Plumbing the sources of endogenous MHC class I peptide ligands. Curr. Opin. Immunol. 19, 79–86 (2007).
Reits, E. et al. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity 20, 495–506 (2004).
York, I.A. et al. The cytosolic endopeptidase, thimet oligopeptidase, destroys antigenic peptides and limits the extent of MHC class I antigen presentation. Immunity 18, 429–440 (2003).
Saric, T., Graef, C.I. & Goldberg, A.L. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J. Biol. Chem. 279, 46723–46732 (2004).
Saveanu, L. et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 6, 689–697 (2005).
Craiu, A., Akopian, T., Goldberg, A. & Rock, K.L. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc. Natl. Acad. Sci. USA 94, 10850–10855 (1997).
Stoltze, L. et al. Generation of the vesicular stomatitis virus nucleoprotein cytotoxic T lymphocyte epitope requires proteasome-dependent and -independent proteolytic activities. Eur. J. Immunol. 28, 4029–4036 (1998).
Mo, X.Y., Cascio, P., Lemerise, K., Goldberg, A.L. & Rock, K. Distinct proteolytic processes generate the C and N termini of MHC class I-binding peptides. J. Immunol. 163, 5851–5859 (1999).
Benham, A.M., Gromme, M. & Neefjes, J. Allelic differences in the relationship between proteasome activity and MHC class I peptide loading. J. Immunol. 161, 83–89 (1998).
Luckey, C.J. et al. Proteasomes can either generate or destroy MHC class I epitopes: evidence for nonproteasomal epitope generation in the cytosol. J. Immunol. 161, 112–121 (1998).
Schwarz, K. et al. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses. J. Immunol. 164, 6147–6157 (2000).
Luckey, C.J. et al. Differences in the expression of human class I MHC alleles and their associated peptides in the presence of proteasome inhibitors. J. Immunol. 167, 1212–1221 (2001).
Marcilla, M., Cragnolini, J.J. & Lopez de Castro, J.A. Proteasome-independent HLA-B27 ligands arise mainly from small basic proteins. Mol. Cell. Proteomics 6, 923–938 (2007).
Seifert, U. et al. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat. Immunol. 4, 375–379 (2003).
Parmentier, N. et al. Production of an antigenic peptide by insulin-degrading enzyme. Nat. Immunol. 11, 449–454 (2010).
van Endert, P. Role of tripeptidyl peptidase II in MHC class I antigen processing - the end of controversies? Eur. J. Immunol. 38, 609–613 (2008).
Tenzer, S. et al. Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cell. Mol. Life Sci. 62, 1025–1037 (2005).
Kessler, J.H. et al. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J. Exp. Med. 193, 73–88 (2001).
Geier, E. et al. A giant protease with potential to substitute for some functions of the proteasome. Science 283, 978–981 (1999).
Fumagalli, P. et al. Human NRD convertase: a highly conserved metalloendopeptidase expressed at specific sites during development and in adult tissues. Genomics 47, 238–245 (1998).
Chow, K.M. et al. Studies on the subsite specificity of rat nardilysin (N-arginine dibasic convertase). J. Biol. Chem. 275, 19545–19551 (2000).
Chow, K.M. et al. Nardilysin cleaves peptides at monobasic sites. Biochemistry 42, 2239–2244 (2003).
Knight, C.G., Dando, P.M. & Barrett, A.J. Thimet oligopeptidase specificity: evidence of preferential cleavage near the C-terminus and product inhibition from kinetic analysis of peptide hydrolysis. Biochem. J. 308, 145–150 (1995).
Oliveira, V. et al. Temperature and salts effects on the peptidase activities of the recombinant metallooligopeptidases neurolysin and thimet oligopeptidase. Eur. J. Biochem. 269, 4326–4334 (2002).
Oliveira, V. et al. Substrate specificity characterization of recombinant metallo oligo-peptidases thimet oligopeptidase and neurolysin. Biochemistry 40, 4417–4425 (2001).
van Swieten, P.F. et al. A cell-permeable inhibitor and activity-based probe for the caspase-like activity of the proteasome. Bioorg. Med. Chem. Lett. 17, 3402–3405 (2007).
Altfeld, M. et al. HLA Alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med. 3, e403 (2006).
Brooks, J.M., Murray, R.J., Thomas, W.A., Kurilla, M.G. & Rickinson, A.B. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J. Exp. Med. 178, 879–887 (1993).
Sigman, J.A. et al. Flexibility in substrate recognition by thimet oligopeptidase as revealed by denaturation studies. Biochem. J. 388, 255–261 (2005).
Kawakami, Y. et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 180, 347–352 (1994).
Romero, P. et al. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201-restricted Melan-A/MART-1 antigenic peptide in melanoma. J. Immunol. 159, 2366–2374 (1997).
Le Gall, S., Stamegna, P. & Walker, B.D. Portable flanking sequences modulate CTL epitope processing. J. Clin. Invest. 117, 3563–3575 (2007).
Schneider, J., Brichard, V., Boon, T., Meyer zum Buschenfelde, K.H. & Wolfel, T. Overlapping peptides of melanocyte differentiation antigen Melan-A/MART- 1 recognized by autologous cytolytic T lymphocytes in association with HLA-B45.1 and HLA-A2.1. Int. J. Cancer 75, 451–458 (1998).
Silva, C.L., Portaro, F.C., Bonato, V.L., de Camargo, A.C. & Ferro, E.S. Thimet oligopeptidase (EC 3.4.24.15), a novel protein on the route of MHC class I antigen presentation. Biochem. Biophys. Res. Commun. 255, 591–595 (1999).
Portaro, F.C. et al. Thimet oligopeptidase and the stability of MHC class I epitopes in macrophage cytosol. Biochem. Biophys. Res. Commun. 255, 596–601 (1999).
Saric, T. et al. Major histocompatibility complex class I-presented antigenic peptides are degraded in cytosolic extracts primarily by thimet oligopeptidase. J. Biol. Chem. 276, 36474–36481 (2001).
Kim, S.I., Pabon, A., Swanson, T.A. & Glucksman, M.J. Regulation of cell-surface major histocompatibility complex class I expression by the endopeptidase EC3.4.24.15 (thimet oligopeptidase). Biochem. J. 375, 111–120 (2003).
Berti, D.A. et al. Analysis of intracellular substrates and products of thimet oligopeptidase (EC 3.4.24.15) in human embryonic kidney 293 cells. J. Biol. Chem. 284, 14105–14116 (2009).
Herberts, C.A. et al. Cutting edge: HLA-B27 acquires Many N-terminal dibasic peptides: coupling cytosolic peptide stability to antigen presentation. J. Immunol. 176, 2697–2701 (2006).
Csuhai, E., Chen, G. & Hersh, L.B. Regulation of N-arginine dibasic convertase activity by amines: putative role of a novel acidic domain as an amine binding site. Biochemistry 37, 3787–3794 (1998).
Mulder, A. et al. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol. Immunol. 47, 809–815 (2010).
Kessler, J.H. et al. Competition-based cellular peptide binding assays for 13 prevalent HLA class I alleles using fluorescein-labeled synthetic peptides. Hum. Immunol. 64, 245–255 (2003).
Kessler, B.M. et al. Extended peptide-based inhibitors efficiently target the proteasome and reveal overlapping specificities of the catalytic beta-subunits. Chem. Biol. 8, 913–929 (2001).
Balow, R.M., Tomkinson, B., Ragnarsson, U. & Zetterqvist, O. Purification, substrate specificity, and classification of tripeptidyl peptidase II. J. Biol. Chem. 261, 2409–2417 (1986).
Tomkinson, B. & Zetterqvist, O. Immunological cross-reactivity between human tripeptidyl peptidase II and fibronectin. Biochem. J. 267, 149–154 (1990).
Roelse, J., Gromme, M., Momburg, F., Hammerling, G. & Neefjes, J. Trimming of TAP-translocated peptides in the endoplasmic reticulum and in the cytosol during recycling. J. Exp. Med. 180, 1591–1597 (1994).
Neisig, A. et al. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I- presentable peptides and the effect of flanking sequences. J. Immunol. 154, 1273–1279 (1995).
Androlewicz, M.J. & Cresswell, P. Human transporters associated with antigen processing possess a promiscuous peptide-binding site. Immunity 1, 7–14 (1994).
Acknowledgements
We thank T. Dannenberg and K. Textoris-Taube for technical assistance. Supported by the Dutch Cancer Society (UL 2005-3245) and Stichting Vanderes.
Author information
Authors and Affiliations
Contributions
J.H.K. conceived of the study, coordinated the work, designed, did and analyzed most experiments and wrote the manuscript with major input from C.J.M.M. and minor input from other authors; C.J.M.M., F.O. and P.A.v.V. provided intellectual input; S.K. did the experiments and analyses in Figure 2b and immunoblot analysis; K.M.C., L.B.H. and A. Prat contributed to the experiments about nardilysin; D.W.R., K.R., U.S. and A. Paschen contributed to the experiments about TOP; P.M.K., U.S. and B.T. contributed to the experiments about TPPII; H.S.O. and P.F.v.S. contributed to the experiments about the proteasome; J.N. and T.v.H. contributed to the experiments about TAP; N.v.M., U.S., A. Paschen, S.L.G. and J.M.B. contributed to CTL experiments; J.W.D., F.O., J.N., S.K. and W.E.B. contributed to substrate design and synthesis; S.A.B.-V. and K.L.M.C.F. contributed to the molecular biology; A.M. and I.I.N.D. made HLA class I mAbs and cell lines expressing a single HLA class I allele; and P.A.v.V. and A.d.R. did mass spectrometry.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–2 (PDF 3479 kb)
Rights and permissions
About this article
Cite this article
Kessler, J., Khan, S., Seifert, U. et al. Antigen processing by nardilysin and thimet oligopeptidase generates cytotoxic T cell epitopes. Nat Immunol 12, 45–53 (2011). https://doi.org/10.1038/ni.1974
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1974
This article is cited by
-
Immunogenicity of necrotic cell death
Cellular and Molecular Life Sciences (2015)
-
Role of peptide processing predictions in T cell epitope identification: contribution of different prediction programs
Immunogenetics (2015)
-
Critical roles of nardilysin in the maintenance of body temperature homoeostasis
Nature Communications (2014)
-
The Aeromonas salmonicida subsp. salmonicida exoproteome: determination of the complete repertoire of Type-Three Secretion System effectors and identification of other virulence factors
Proteome Science (2013)
-
Serpinb9 (Spi6)‐deficient mice are impaired in dendritic cell‐mediated antigen cross‐presentation
Immunology & Cell Biology (2012)