Abstract
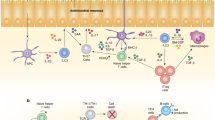
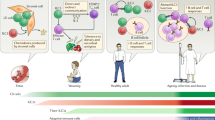
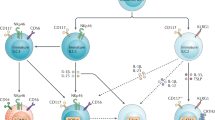
Research has identified what can be considered a family of innate lymphoid cells (ILCs) that includes not only natural killer (NK) cells and lymphoid tissue–inducer (LTi) cells but also cells that produce interleukin 5 (IL-5), IL-13, IL-17 and/or IL-22. These ILC subsets are developmentally related, requiring expression of the transcriptional repressor Id2 and cytokine signals through the common γ-chain of the IL-2 receptor. The functional differentiation of ILC subsets is orchestrated by distinct transcription factors. Analogous to helper T cell subsets, these evolutionarily conserved yet distinct ILCs seem to have important roles in protective immunity, and their dysregulation can promote immune pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Biron, C.A., Nguyen, K.B., Pien, G.C., Cousens, L.P. & Salazar-Mather, T.P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Arase, H. & Lanier, L.L. Virus-driven evolution of natural killer cell receptors. Microbes Infect. 4, 1505–1512 (2002).
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Di Santo, J.P. Natural killer cells: diversity in search of a niche. Nat. Immunol. 9, 473–475 (2008).
Lanier, L.L., Le, A.M., Civin, C.I., Loken, M.R. & Phillips, J.H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol. 136, 4480–4486 (1986).
Cooper, M.A., Fehniger, T.A. & Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
Fauriat, C., Long, E.O., Ljunggren, H.G. & Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115, 2167–2176 (2010).
Hayakawa, Y., Huntington, N.D., Nutt, S.L. & Smyth, M.J. Functional subsets of mouse natural killer cells. Immunol. Rev. 214, 47–55 (2006).
Vosshenrich, C.A. et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7, 1217–1224 (2006).
Ribeiro, V.S. et al. Thymic NK Cells develop independently from T cell precursors. J. Immunol. 185, 4993–4997 (2010).
Huntington, N.D., Vosshenrich, C.A. & Di Santo, J.P. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7, 703–714 (2007).
Ashkar, A.A., Di Santo, J.P. & Croy, B.A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 192, 259–270 (2000).
Gur, C. et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat. Immunol. 11, 121–128 (2010).
Mebius, R.E., Rennert, P. & Weissman, I.L. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
van de Pavert, S.A. & Mebius, R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
Nishikawa, S.-I., Honda, K., Vieira, P. & Yoshida, H. Organogenesis of peripheral lymphoid organs. Immunol. Rev. 195, 72–80 (2003).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Eberl, G. et al. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17, 823–833 (2002).
Cupedo, T. et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nat. Immunol. 10, 66–74 (2009).
Schmutz, S. et al. Cutting edge: IL-7 regulates the peripheral pool of adult RORγ+ lymphoid tissue inducer cells. J. Immunol. 183, 2217–2221 (2009).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Scandella, E. et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 9, 667–675 (2008).
Ouyang, W., Kolls, J.K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Takatori, H. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41 (2009).
Blaho, V.A., Buczynski, M.W., Dennis, E.A. & Brown, C.R. Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. J. Immunol. 183, 5644–5653 (2009).
Hsu, H.C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 9, 166–175 (2008).
Wolk, K., Witte, E., Witte, K., Warszawska, K. & Sabat, R. Biology of interleukin-22. Semin. Immunopathol. 32, 17–31 (2010).
Tsuji, M. et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008).
Lane, P. et al. Lymphoid tissue inducer cells in adaptive CD4 T cell dependent responses. Semin. Immunol. 20, 159–163 (2008).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Sanos, S.L. et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Satoh-Takayama, N. et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3−NKp46+ cell subsets from Id2-dependent precursors. J. Exp. Med. 207, 273–280 (2010).
Crellin, N.K., Trifari, S., Kaplan, C.D., Cupedo, T. & Spits, H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 207, 281–290 (2010).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Satoh-Takayama, N. et al. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J. Immunol. 183, 6579–6587 (2009).
Crellin, N. et al. Regulation of cytokine secretion in human CD127+ LTi-like innate lymphoid cells by Toll like receptor 2. Immunity doi:10.1016/j.immuni.2010.10.012 (4 November 2010).
Cella, M., Otero, K. & Colonna, M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc. Natl. Acad. Sci. USA 107, 10961–10966 (2010).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Fort, M.M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Hurst, S.D. et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169, 443–453 (2002).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Neill, D.R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Saenz, S.A. et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464, 1362–1366 (2010).
Fallon, P.G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Owyang, A.M. et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 (2006).
Price, A.E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107, 11489–11494 (2010).
Loza, M.J., Zamai, L., Azzoni, L., Rosati, E. & Perussia, B. Expression of type 1 (interferon γ) and type 2 (interleukin-13, interleukin-5) cytokines at distinct stages of natural killer cell differentiation from progenitor cells. Blood 99, 1273–1281 (2002).
Veldhoen, M. et al. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9, 1341–1346 (2008).
Mukasa, R. et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32, 616–627 (2010).
O'Shea, J.J. & Paul, W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102 (2010).
Kee, B.L. E and ID proteins branch out. Nat. Rev. Immunol. 9, 175–184 (2009).
Boos, M.D., Yokota, Y., Eberl, G. & Kee, B.L. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130 (2007).
Heemskerk, M.H. et al. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J. Exp. Med. 186, 1597–1602 (1997).
Spits, H., Couwenberg, F., Bakker, A.Q., Weijer, K. & Uittenbogaart, C.H. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192, 1775–1784 (2000).
Gascoyne, D.M. et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat. Immunol. 10, 1118–1124 (2009).
Kamizono, S. et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J. Exp. Med. 206, 2977–2986 (2009).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Trifari, S., Kaplan, C.D., Tran, E.H., Crellin, N.K. & Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 10, 864–871 (2009).
Wincent, E. et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J. Biol. Chem. 284, 2690–2696 (2009).
Wilkinson, B. et al. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat. Immunol. 3, 272–280 (2002).
Aliahmad, P., de la Torre, B. & Kaye, J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat. Immunol. 11, 945–952 (2010).
Burkett, P.R. et al. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200, 825–834 (2004).
Koka, R. et al. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J. Exp. Med. 197, 977–984 (2003).
Mortier, E., Woo, T., Advincula, R., Gozalo, S. & Ma, A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205, 1213–1225 (2008).
Puel, A., Ziegler, S.F., Buckley, R.H. & Leonard, W.J. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat. Genet. 20, 394–397 (1998).
Huntington, N.D. et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 206, 25–34 (2009).
Freud, A.G. et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22, 295–304 (2005).
Freud, A.G. et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J. Exp. Med. 203, 1033–1043 (2006).
Hughes, T. et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH17 cytokine interleukin-22. Blood 113, 4008–4010 (2009).
Hughes, T. et al. Interleukin-1β selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 32, 803–814 (2010).
Ahern, P.P. et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 (2010).
Uhlig, H.H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Duerr, R.H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Eisenring, M., Vom Berg, J., Kristiansen, G., Saller, E. & Becher, B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat. Immunol. 11, 1030–1038 (2010).
Lane, P.J.L., Gaspal, F.M.C. & Kim, M.-Y. Two sides of a cellular coin: CD4+CD3− cells regulate memory responses and lymph-node organization. Nat. Rev. Immunol. 5, 655–660 (2005).
Khalturin, K., Panzer, Z., Cooper, M.D. & Bosch, T.C. Recognition strategies in the innate immune system of ancestral chordates. Mol. Immunol. 41, 1077–1087 (2004).
Acknowledgements
We thank T. Cupedo, N. Crellin, S. Trifari and C. Kaplan for contributions and collaborations; and G. Eberl, N. Satoh-Takayama and C. Vosshenrich for collaborations. Supported by the Institut Pasteur (J.P.D.), Institut National de la Santé et de la Recherche Médicale (J.P.D.) and the Agence National de Recherches (J.P.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Spits, H., Di Santo, J. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12, 21–27 (2011). https://doi.org/10.1038/ni.1962
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1962
This article is cited by
-
Colonization and development of the gut microbiome in calves
Journal of Animal Science and Biotechnology (2023)
-
Intragraft immune cells: accomplices or antagonists of recipient-derived macrophages in allograft fibrosis?
Cellular and Molecular Life Sciences (2023)
-
RORγt agonist enhances anti-PD-1 therapy by promoting monocyte-derived dendritic cells through CXCL10 in cancers
Journal of Experimental & Clinical Cancer Research (2022)
-
Keeping ILCs in shape: PD-1 as a metabolic checkpoint
Nature Metabolism (2022)
-
Innate lymphoid cell dysfunction during long-term suppressive antiretroviral therapy in an African cohort
BMC Immunology (2021)