Abstract
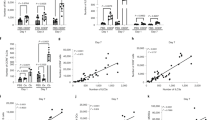
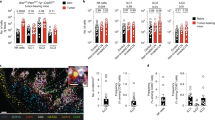
The potent tumoricidal activity of interleukin 12 (IL-12) is thought to be mediated by the activation and polarization of natural killer (NK) cells and T helper type 1 (TH1) cells, respectively. By systematic analysis of the IL-12-induced immune response to subcutaneous melanoma (B16), we found that tumor suppression was mediated independently of T lymphocytes or NK cells. IL-12 initiated local antitumor immunity by stimulating a subset of NKp46+ lymphoid tissue–inducer (LTi) cells dependent on the transcription factor RORγt. The presence of these NKp46+ LTi cells induced upregulation of adhesion molecules in the tumor vasculature and resulted in more leukocyte invasion. Thus, this innate cell type is responsive to IL-12 and is a powerful mediator of tumor suppression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dunn, G.P., Koebel, C.M. & Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Kinlen, L.J., Sheil, A.G., Peto, J. & Doll, R. Collaborative United Kingdom-Australasian study of cancer in patients treated with immunosuppressive drugs. BMJ 2, 1461–1466 (1979).
Buell, J.F., Gross, T.G. & Woodle, E.S. Malignancy after transplantation. Transplantation 80, S254–S264 (2005).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Dunn, G.P., Old, L.J. & Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6, 715–727 (2006).
Schoenhaut, D.S. et al. Cloning and expression of murine IL-12. J. Immunol. 148, 3433–3440 (1992).
Trinchieri, G. & Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7, 179–190 (2007).
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003).
Nishimura, T. et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J. Exp. Med. 190, 617–627 (1999).
Nastala, C.L. et al. Recombinant IL-12 administration induces tumor regression in association with IFN-γ production. J. Immunol. 153, 1697–1706 (1994).
Tahara, H. et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 54, 182–189 (1994).
Noguchi, Y., Jungbluth, A., Richards, E.C. & Old, L.J. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc. Natl. Acad. Sci. USA 93, 11798–11801 (1996).
Del Vecchio, M. et al. Interleukin-12: biological properties and clinical application. Clin. Cancer Res. 13, 4677–4685 (2007).
Langowski, J.L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465 (2006).
Brunda, M.J. et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 178, 1223–1230 (1993).
Smyth, M.J., Taniguchi, M. & Street, S.E. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J. Immunol. 165, 2665–2670 (2000).
Smyth, M.J. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 (2000).
Smyth, M.J., Hayakawa, Y., Takeda, K. & Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2, 850–861 (2002).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Kodama, T. et al. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur. J. Immunol. 29, 1390–1396 (1999).
Park, S.H., Kyin, T., Bendelac, A. & Carnaud, C. The contribution of NKT cells, NK cells, and other γ-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J. Immunol. 170, 1197–1201 (2003).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Sanos, S.L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Cupedo, T. et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nat. Immunol. 10, 66–74 (2009).
Vivier, E., Spits, H. & Cupedo, T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat. Rev. Immunol. 9, 229–234 (2009).
Belladonna, M.L. et al. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168, 5448–5454 (2002).
Schmidt, S.R. Fusion-proteins as biopharmaceuticals–applications and challenges. Curr. Opin. Drug Discov. Dev. 12, 284–295 (2009).
Vosshenrich, C.A. et al. Roles for common cytokine receptor γ-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 174, 1213–1221 (2005).
Beadling, C. & Slifka, M.K. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch. Immunol. Ther. Exp. (Warsz.) 54, 15–24 (2006).
Kim, M.Y. et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J. Immunol. 174, 6686–6691 (2005).
Walzer, T. et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 104, 3384–3389 (2007).
Satoh-Takayama, N. et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3−NKp46+ cell subsets from Id2-dependent precursors. J. Exp. Med. 207, 273–280 (2009).
Crellin, N.K., Trifari, S., Kaplan, C.D., Cupedo, T. & Spits, H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 207, 281–290 (2010).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Eberl, G. et al. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Takatori, H. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41 (2009).
Schmutz, S. et al. Cutting edge: IL-7 regulates the peripheral pool of adult RORγ+ lymphoid tissue inducer cells. J. Immunol. 183, 2217–2221 (2009).
Ryschich, E., Schmidt, J., Hammerling, G.J., Klar, E. & Ganss, R. Transformation of the microvascular system during multistage tumorigenesis. Int. J. Cancer 97, 719–725 (2002).
Buckanovich, R.J. et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 14, 28–36 (2008).
Lugade, A.A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Quezada, S.A. et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J. Exp. Med. 205, 2125–2138 (2008).
Schrama, D. et al. Targeting of lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 14, 111–121 (2001).
Sun, J.C., Ma, A. & Lanier, L.L. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J. Immunol. 183, 2911–2914 (2009).
Shields, J.D., Kourtis, I.C., Tomei, A.A., Roberts, J.M. & Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Marshall, E. Sciencescope. Science 268, 1555 (1995).
Daud, A.I. et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 26, 5896–5903 (2008).
Peggs, K.S., Quezada, S.A., Chambers, C.A., Korman, A.J. & Allison, J.P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Acknowledgements
We thank S. Bulfone-Paus (Research Center Borstel) for Il15ra−/− mice; A. Diefenbach (University Hospital Freiburg) for Rorc-eYFP mice; Y. Iwakura (University of Tokyo) for Il17a−/− mice; J.C. Renauld (Ludwig Institute Brussels) for Il22−/− mice; R. Zinkernagel (University Hospital of Zurich) for anti-CD4 and anti-CD8; M. Kopf and A. Diefenbach for discussions; and V. Tosevski, S. Burkhard, B. Sobotka, V. Wortmann and S. Behnke for technical assistance. Supported by the Swiss National Science Foundation (B.B.), the Swiss Cancer League (B.B.), the Center for Neurosciences Zurich (M.E.) and the Marie Heim-Vögtlin Programme (E.S.).
Author information
Authors and Affiliations
Contributions
M.E. and E.S. designed and did the experiments and analyzed the data; J.v.B. did experiments and analyzed the histology; G.K. provided advice for the histological analysis; B.B. designed the experiments, supervised and funded the study; B.B. and M.E. wrote the report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 391 kb)
Rights and permissions
About this article
Cite this article
Eisenring, M., vom Berg, J., Kristiansen, G. et al. IL-12 initiates tumor rejection via lymphoid tissue–inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol 11, 1030–1038 (2010). https://doi.org/10.1038/ni.1947
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1947
This article is cited by
-
Innate lymphoid cells and innate-like T cells in cancer — at the crossroads of innate and adaptive immunity
Nature Reviews Cancer (2023)
-
Evaluation of circulating innate lymphoid cells in the early pathogenesis of mouse colorectal carcinoma
Comparative Clinical Pathology (2023)
-
T cell-independent eradication of experimental glioma by intravenous TLR7/8-agonist-loaded nanoparticles
Nature Communications (2023)
-
Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses
Nature Immunology (2022)
-
Plasticity of innate lymphoid cell subsets
Nature Reviews Immunology (2020)