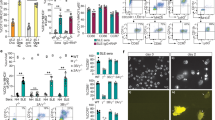
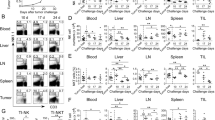
Abstract
Neutrophils are the main effector cells during inflammation, but they can also control excessive inflammatory responses by secreting anti-inflammatory cytokines. However, the mechanisms that modulate their plasticity remain unclear. We now show that systemic serum amyloid A 1 (SAA-1) controls the plasticity of neutrophil differentiation. SAA-1 not only induced anti-inflammatory interleukin 10 (IL-10)-secreting neutrophils but also promoted the interaction of invariant natural killer T cells (iNKT cells) with those neutrophils, a process that limited their suppressive activity by diminishing the production of IL-10 and enhancing the production of IL-12. Because SAA-1-producing melanomas promoted differentiation of IL-10-secreting neutrophils, harnessing iNKT cells could be useful therapeutically by decreasing the frequency of immunosuppressive neutrophils and restoring tumor-specific immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Martinez, F.O., Helming, L. & Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 (2009).
Gabrilovich, D.I. et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 67, 425 (2007).
Youn, J.I., Nagaraj, S., Collazo, M. & Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008).
De Santo, C. et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl. Acad. Sci. USA 102, 4185–4190 (2005).
Fridlender, Z.G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009).
Zhang, X., Majlessi, L., Deriaud, E., Leclerc, C. & Lo-Man, R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31, 761–771 (2009).
Cross, A., Edwards, S.W., Bucknall, R.C. & Moots, R.J. Secretion of oncostatin M by neutrophils in rheumatoid arthritis. Arthritis Rheum. 50, 1430–1436 (2004).
Urieli-Shoval, S., Linke, R.P. & Matzner, Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr. Opin. Hematol. 7, 64–69 (2000).
Jacobs, D.M. & Morrison, D.C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J. Immunol. 118, 21–27 (1977).
He, R.L. et al. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 113, 429–437 (2009).
Dufton, N. et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 184, 2611–2619 (2010).
Ye, R.D. et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 (2009).
Cheng, N., He, R., Tian, J., Ye, P.P. & Ye, R.D. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 181, 22–26 (2008).
He, R., Sang, H. & Ye, R.D. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 101, 1572–1581 (2003).
He, R., Shepard, L.W., Chen, J., Pan, Z.K. & Ye, R.D. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J. Immunol. 177, 4072–4079 (2006).
Sander, L.E. et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 207, 1453–1464 (2010).
De Santo, C. et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J. Clin. Invest. 118, 4036–4048 (2008).
Brigl, M., Bry, L., Kent, S.C., Gumperz, J.E. & Brenner, M.B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003).
Salio, M. et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. USA 104, 20490–20495 (2007).
Paget, C. et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007).
Godfrey, D.I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Salio, M. & Cerundolo, V. Linking inflammation to natural killer T cell activation. PLoS Biol. 7, e1000226 (2009).
Hermans, I.F. et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 171, 5140–5147 (2003).
Silk, J.D. et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Invest. 114, 1800–1811 (2004).
Galli, G. et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. USA 104, 3984–3989 (2007).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Raghuraman, G., Geng, Y. & Wang, C.R. IFN-β-mediated up-regulation of CD1d in bacteria-infected APCs. J. Immunol. 177, 7841–7848 (2006).
Skold, M., Xiong, X., Illarionov, P.A., Besra, G.S. & Behar, S.M. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J. Immunol. 175, 3584–3593 (2005).
Morris, E.S. et al. Induction of natural killer T cell-dependent alloreactivity by administration of granulocyte colony-stimulating factor after bone marrow transplantation. Nat. Med. 15, 436–441 (2009).
Fox, L.M. et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7, e1000228 (2009).
Matsuda, J.L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Godfrey, D.I., MacDonald, H.R., Kronenberg, M., Smyth, M.J. & Van Kaer, L. NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Pellicci, D.G. et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195, 835–844 (2002).
Crowe, N.Y. et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202, 1279–1288 (2005).
Coquet, J.M. et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4–NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA 105, 11287–11292 (2008).
Michel, M.L. et al. Identification of an IL-17-producing NK1.1− iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 (2007).
Doisne, J.M. et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor γt+ and respond preferentially under inflammatory conditions. J. Immunol. 183, 2142–2149 (2009).
Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116, 1218–1222 (2006).
Michel, M.L. et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc. Natl. Acad. Sci. USA 105, 19845–19850 (2008).
Rachitskaya, A.V. et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180, 5167–5171 (2008).
Tahir, S.M. et al. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J. Immunol. 167, 4046–4050 (2001).
Dhodapkar, M.V. et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 197, 1667–1676 (2003).
Molling, J.W. et al. Peripheral blood IFN-γ-secreting Vα24+Vβ11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int. J. Cancer 116, 87–93 (2005).
van der Vliet, H.J. et al. Circulating myeloid dendritic cells of advanced cancer patients result in reduced activation and a biased cytokine profile in invariant NKT cells. J. Immunol. 180, 7287–7293 (2008).
Queen, M.M., Ryan, R.E., Holzer, R.G., Keller-Peck, C.R. & Jorcyk, C.L. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 65, 8896–8904 (2005).
Wislez, M. et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 63, 1405–1412 (2003).
Rodriguez, P.C. et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560 (2009).
Findeisen, P. et al. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J. Clin. Oncol. 27, 2199–2208 (2009).
Paret, C., Schon, Z., Szponar, A. & Kovacs, G. Inflammatory protein serum amyloid A1 marks a subset of conventional renal cell carcinomas with fatal outcome. Eur. Urol. 57, 859–866 (2009).
Hiratsuka, S. et al. The S100A8-serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Uhlar, C.M. & Whitehead, A.S. The kinetics and magnitude of the synergistic activation of the serum amyloid A promoter by IL-1β and IL-6 is determined by the order of cytokine addition. Scand. J. Immunol. 49, 399–404 (1999).
Uhlar, C.M. & Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 265, 501–523 (1999).
Whicher, J.T., Chambers, R.E., Higginson, J., Nashef, L. & Higgins, P.G. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. J. Clin. Pathol. 38, 312–316 (1985).
Dunbar, P.R. et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J. Immunol. 165, 6644–6652 (2000).
Karadimitris, A. et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc. Natl. Acad. Sci. USA 98, 3294–3298 (2001).
Corzo, C.A. et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 (2009).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Mendiratta, S.K. et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 (1997).
Salio, M. et al. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J. Immunol. 167, 1188–1197 (2001).
Acknowledgements
We thank L. Van Kaer (Vanderbilt University School of Medicine) for Cd1d−/− (CD45.2+/+) mice; R. Lisle and K. Hollowood for help in preparing melanoma sample sections and analyzing stained samples; G. Napolitani, A. Stock and M. Johnson for discussions and critical reading of the manuscript; and P. Polzella for technical support. Supported by Cancer Research UK (C399/A2291), the UK Medical Research Council, The Wellcome Trust (084923/Z/08/Z), the Cancer Research Institute, the Ludwig Institute for Cancer Research (V.C.), the Oxford National Institute for Health Research Biomedical Research Centre (M.M., R. Asher and M.J.) and the Oxford Experimental Cancer Medicine Centre (for clinical sample processing).
Author information
Authors and Affiliations
Contributions
C.D.S. designed and did the experiments, prepared the figures and contributed to the writing of the manuscript; R. Arscott did specific experiments; I.K. and M.M. obtained consent from patients with melanoma and collected blood samples; S.B., M.J. and R. Asher did tissue staining of melanoma sections; M.S. provided reagents and contributed to the writing of the manuscript; and V.C. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 815 kb)
Rights and permissions
About this article
Cite this article
De Santo, C., Arscott, R., Booth, S. et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol 11, 1039–1046 (2010). https://doi.org/10.1038/ni.1942
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1942
This article is cited by
-
Massively recruited sTLR9+ neutrophils in rapidly formed nodules at the site of tumor cell inoculation and their contribution to a pro-tumor microenvironment
Cancer Immunology, Immunotherapy (2023)
-
Invariant NKT cells metabolically adapt to the acute myeloid leukaemia environment
Cancer Immunology, Immunotherapy (2023)
-
Sportomics method to assess acute phase proteins in Olympic level athletes using dried blood spots and multiplex assay
Scientific Reports (2022)
-
Role of NKT cells in cancer immunotherapy—from bench to bed
Medical Oncology (2022)
-
IFN-γ-dependent NK cell activation is essential to metastasis suppression by engineered Salmonella
Nature Communications (2021)