Abstract
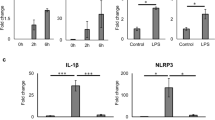
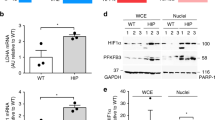
Interleukin 1β (IL-1β) is an important inflammatory mediator of type 2 diabetes. Here we show that oligomers of islet amyloid polypeptide (IAPP), a protein that forms amyloid deposits in the pancreas during type 2 diabetes, triggered the NLRP3 inflammasome and generated mature IL-1β. One therapy for type 2 diabetes, glyburide, suppressed IAPP-mediated IL-1β production in vitro. Processing of IL-1β initiated by IAPP first required priming, a process that involved glucose metabolism and was facilitated by minimally oxidized low-density lipoprotein. Finally, mice transgenic for human IAPP had more IL-1β in pancreatic islets, which localized together with amyloid and macrophages. Our findings identify previously unknown mechanisms in the pathogenesis of type 2 diabetes and treatment of pathology caused by IAPP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kahn, S.E., Hull, R.L. & Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Njajou, O.T. et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab. Res. Rev. 25, 733–739 (2009).
Bendtzen, K. et al. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232, 1545–1547 (1986).
Spranger, J. et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52, 812–817 (2003).
Larsen, C.M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Clark, A. et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 9, 151–159 (1988).
Cooper, G.J. et al. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. USA 84, 8628–8632 (1987).
Westermark, P. et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl. Acad. Sci. USA 84, 3881–3885 (1987).
Butler, A.E. et al. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes 53, 1509–1516 (2004).
Janson, J. et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc. Natl. Acad. Sci. USA 93, 7283–7288 (1996).
Verchere, C.B. et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc. Natl. Acad. Sci. USA 93, 3492–3496 (1996).
Howard, C.F. Jr. Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia 29, 301–306 (1986).
Seino, S. S20G mutation of the amylin gene is associated with type II diabetes in Japanese. Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus. Diabetologia 44, 906–909 (2001).
Ma, Z. et al. Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid 8, 242–249 (2001).
Udayasankar, J. et al. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia 52, 145–153 (2009).
Westermark, P., Eizirik, D.L., Pipeleers, D.G., Hellerstrom, C. & Andersson, A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia 38, 543–549 (1995).
Westermark, G.T., Westermark, P., Berne, C. & Korsgren, O. Widespread amyloid deposition in transplanted human pancreatic islets. N. Engl. J. Med. 359, 977–979 (2008).
Lorenzo, A., Razzaboni, B., Weir, G.C. & Yankner, B.A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368, 756–760 (1994).
Janson, J., Ashley, R.H., Harrison, D., McIntyre, S. & Butler, P.C. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes 48, 491–498 (1999).
Zraika, S. et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 52, 626–635 (2009).
Zraika, S. et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia 53, 1046–1056 (2010).
Badman, M.K., Pryce, R.A., Charge, S.B., Morris, J.F. & Clark, A. Fibrillar islet amyloid polypeptide (amylin) is internalised by macrophages but resists proteolytic degradation. Cell Tissue Res. 291, 285–294 (1998).
de Koning, E.J. et al. Macrophages and pancreatic islet amyloidosis. Amyloid 5, 247–254 (1998).
Gitter, B.D., Cox, L.M., Carlson, C.D. & May, P.C. Human amylin stimulates inflammatory cytokine secretion from human glioma cells. Neuroimmunomodulation 7, 147–152 (2000).
Yates, S.L. et al. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 74, 1017–1025 (2000).
Lamkanfi, M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Sharp, F.A. et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 106, 870–875 (2009).
Keller, M., Ruegg, A., Werner, S. & Beer, H.D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Hay, D.L., Christopoulos, G., Christopoulos, A., Poyner, D.R. & Sexton, P.M. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol. Pharmacol. 67, 1655–1665 (2005).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 (2008).
Hamon, Y. et al. Interleukin-1β secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 90, 2911–2915 (1997).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Boni-Schnetzler, M. et al. Increased interleukin (IL)-1β messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 93, 4065–4074 (2008).
Maedler, K. et al. Glucose-induced beta cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110, 851–860 (2002).
Welsh, N. et al. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 54, 3238–3244 (2005).
Miller, Y.I. et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278, 1561–1568 (2003).
Apolinario, E. et al. Minimally modified (electronegative) LDL– and Anti-LDL– autoantibodies in diabetes mellitus and impaired glucose tolerance. Int. J Atheroscler. 1, 42–47 (2006).
Yano, M. et al. Increased electronegative charge of serum low-density lipoprotein in patients with diabetes mellitus. Clin. Chim. Acta 340, 93–98 (2004).
Abderrahmani, A. et al. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia 50, 1304–1314 (2007).
Bauernfeind, F.G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Matveyenko, A.V. & Butler, P.C. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 47, 225–233 (2006).
Hull, R.L. et al. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes 52, 372–379 (2003).
Butler, A.E., Janson, J., Soeller, W.C. & Butler, P.C. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52, 2304–2314 (2003).
van de Veerdonk, F.L. et al. Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 107, 3030–3033 (2010).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Russell, J.C. & Proctor, S.D. Increased insulin sensitivity and reduced micro and macro vascular disease induced by 2-deoxy-D-glucose during metabolic syndrome in obese JCR: LA-cp rats. Br. J. Pharmacol. 151, 216–225 (2007).
Michalska, M., Wolf, G., Walther, R. & Newsholme, P. The effects of pharmacologic inhibition of NADPH oxidase or iNOS on pro-inflammatory cytokine, palmitic acid or H2O2 -induced mouse islet or clonal pancreatic beta cell dysfunction. Biosci. Rep., published online doi:10.1042/BSR20090138 (23 February 2010).
Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 34, 151–160 (2004).
Wang, F., Hull, R.L., Vidal, J., Cnop, M. & Kahn, S.E. Islet amyloid develops diffusely throughout the pancreas before becoming severe and replacing endocrine cells. Diabetes 50, 2514–2520 (2001).
Acknowledgements
We thank A. Mori for assistance with Nlrp3−/− mice; J. Tschopp (University of Lausanne) for Nlrp3−/− mice; and E. Latz (University of Bonn) for YFP-ASC BMDMs. Supported by the National Health and Medical Research Council (516783 to S.L.M.), Science Foundation Ireland (for work at Trinity College Dublin), the United States Department of Veterans Affairs (for work at the VA Puget Sound Health Care System), the US National Institutes of Health (DK-75998 to S.E.K. for work at VA Puget Sound Health Care System, and AI063331 for work at the University of Michigan) and the Crohn's and Colitis Foundation (L.F.).
Author information
Authors and Affiliations
Contributions
S.L.M. designed and did experiments, analyzed data and wrote the paper; L.A.J.O. and E.C.L. conceived ideas and oversaw research; A.D., S.L.S., R.L.H., G.M.T., F.A.S., C.B., L.F., E.Y., Z.C., N.M., L.A.M., J.H. and R.C.C. did experiments; and K.H.G.M., K.H.M., P.N., G.N., J.Y. and S.E.K. provided advice and reagents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 1913 kb)
Rights and permissions
About this article
Cite this article
Masters, S., Dunne, A., Subramanian, S. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11, 897–904 (2010). https://doi.org/10.1038/ni.1935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1935
This article is cited by
-
The role of NLRP3 inflammasome in aging and age-related diseases
Immunity & Ageing (2024)
-
The E3 ubiquitin ligase MARCH2 protects against myocardial ischemia-reperfusion injury through inhibiting pyroptosis via negative regulation of PGAM5/MAVS/NLRP3 axis
Cell Discovery (2024)
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
-
STING signaling in islet macrophages impairs insulin secretion in obesity
Science China Life Sciences (2024)